Mechanistic impact of organics on electro-induced transformation of iron oxides and simultaneous uranium immobilization in wastewater
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
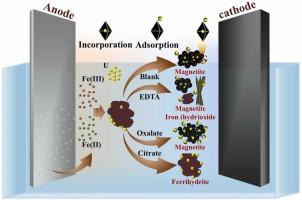
The incorporation of uranium into the magnetite generated through via electrochemical methods represents a sustainable strategy for remediation of uranium-contaminated organic wastewater. Nevertheless, the influence mechanisms of organics on this treatment process remain insufficiently understood. This study used an electrochemical system featuring iron and graphite electrodes along with sodium chloride as the electrolyte to investigate the impact of various organics on uranium removal. The results showed that disodium ethylenediaminetetraacetate addition delayed magnetite formation, resulting in a final product with a mixture of various iron oxides. However, this alteration did not significantly affect the mechanism and efficiency of uranium removal. In contrast, the introduction of oxalate reduced the particle size of magnetite, thereby shifting the primary mechanism of uranium removal towards adsorption, which results in a slight decrease in removal efficiency. Notably, due to the chelation properties of citrate, which nearly eliminate Fe(II) in the solution, magnetite formation was inhibited, thereby substantially reducing the final uranium removal. A 200-day leaching experiment demonstrated that the structural integrity of the synthesized mineral is predominantly maintained. This study elucidates the impact of common organics on the electrochemical mineralization system for uranium removal and offers theoretical guidance for the treatment of uranium-contaminated organic wastewater.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


