Effects of a 12-Week Mediterranean-Type Time-Restricted Feeding Protocol in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease: A Randomised Controlled Trial—The ‘CHRONO-NAFLD Project’
Abstract
Background
The Mediterranean diet (MD) is considered the best dietary approach for patients with metabolic dysfunction-associated steatotic liver disease (MASLD). Recently, time-restricted feeding (TRF) has gained attention for its lifestyle compatibility and health benefits.
Aims
This study aimed to compare the effects of a hypocaloric MD with a 10-h TRF protocol to an unrestricted MD in MASLD patients with overweight/obesity and evaluate differences between early and late TRF.
Methods
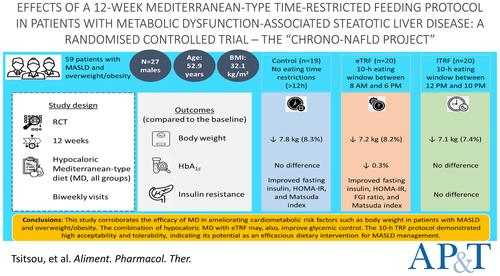
This 12-week randomised controlled trial in MASLD patients with overweight/obesity consisted of three groups, all following a hypocaloric Mediterranean-type diet. The control group had no eating time restrictions. The early TRF (eTRF) and late TRF (lTRF) groups had a 10-h eating window, from 8 AM to 6 PM and from 12 PM to 10 PM, respectively. Various health parameters were measured. Compliance was tracked via food diaries, and an 8-week follow-up occurred post-intervention.
Results
Fifty-nine MASLD individuals (27 males; 52.9 years; body mass index 32.1 kg/m2) completed the trial (control, n = 19; eTRF, n = 20; lTRF, n = 20). All groups showed significant 12-week reductions in body weight, anthropometry and blood pressure. Glycated haemoglobin A1c and insulin resistance, as measured by the Matsuda index, homeostatic model assessment for insulin resistance and fasting glucose-to-insulin ratio, improved in the eTRF group at 12 weeks.
Conclusions
This study corroborates the efficacy of MD in ameliorating cardiometabolic risk factors such as body weight and blood pressure in MASLD patients. The combination with an eTRF protocol may improve glycaemic control (NCT05866744).
Trial Registration
The study is registered at clinicaltrials.gov (NCT05866744)


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


