Role of Basic Sites on Cu/ZrO2 Catalysts Modified with Citric Acid in the Hydrogenation of CO2 to Methanol
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The hydrogenation of CO2 to methanol is one of the most industrially promising measures to solve the excessive emission of CO2, although the development of highly active methanol catalysts is still a challenge. Herein, different modification methods of citric acid in the Cu/ZrO2 catalyst system are compared, and the quantities and strengths of the basic sites of the catalysts could be adjusted by changing the modification method of citric acid. Using various characterization methods, it was found that introducing an appropriate amount of citric acid during the preparation of the support could form a complex with the zirconium dioxide precursor, which can significantly change the distribution of basic sites and the number of hydroxyl groups on the surface of the support. Moreover, the catalyst can generate more oxygen vacancies, enhance the interaction between the metal and the support, and significantly improve the adsorption and activation of the reaction gas. Compared with the unmodified catalyst, the space-time yield of methanol increases from 300 to 660 gMeOH·h–1·kgcat–1 under the reaction conditions of 260 °C and 3.0 MPa. This strategy and the experimental results provide a novel understanding of citric acid modification and the role of basic sites in the conversion of CO2 to methanol.
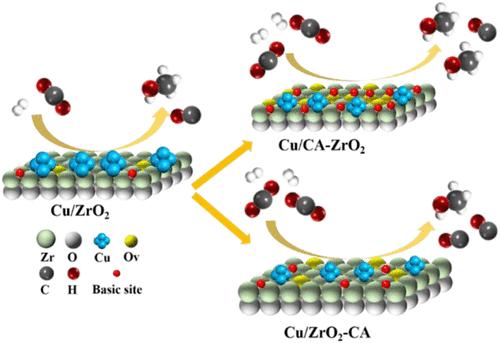
将 CO2 加氢转化为甲醇是解决 CO2 过度排放的最具工业前景的措施之一,但高活性甲醇催化剂的开发仍是一项挑战。本文比较了 Cu/ZrO2 催化剂体系中柠檬酸的不同改性方法,并通过改变柠檬酸的改性方法来调整催化剂碱性位点的数量和强度。通过各种表征方法发现,在制备载体时引入适量的柠檬酸可与二氧化锆前驱体形成络合物,从而显著改变载体表面碱性位点的分布和羟基的数量。此外,催化剂还能产生更多的氧空位,增强金属与载体之间的相互作用,显著提高反应气体的吸附和活化能力。与未改性催化剂相比,在 260 °C 和 3.0 MPa 的反应条件下,甲醇的时空产率从 300 gMeOH-h-1-kgcat-1 增加到 660 gMeOH-h-1-kgcat-1。这一策略和实验结果为柠檬酸改性以及碱性位点在二氧化碳转化为甲醇过程中的作用提供了新的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


