Injectable biodegradable microchamber array films for long-term delivery of glucocorticoids
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
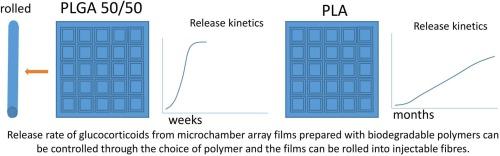
Glucocorticoids (GCs) are widely recognized for their potent anti-inflammatory and analgesic effects. Although they can cause an array of side effects when delivered systemically these are generally avoided when delivered locally at disease sites such as the eyes, lungs and joints. Glucocorticoid formulations for local use range from crystals and particles through to non-biodegradable implants. In many formulations burst release means that their effectiveness does not persist for more than a few weeks. Novel delivery methods that achieve prolonged delivery of GCs along with sequential degradation of the polymer vehicle has the potential to enhance the effectiveness of these drugs and achieve better control of disease. In this study we use a soft lithography method to produce polymer microchamber array films (MCAs) containing crystals of GCs. We demonstrate that the rate of glucocorticoid release can be adjusted through the choice of polymer used in the manufacture of films with rapid release observed with PLGA 50/50 over the course of 9 weeks and the longest duration of release observed with PLA films which continued beyond a year. Importantly, these release studies do not show evidence of burst release and all films displayed a significant duration of zero order release kinetics. Observations of film degradation were made through changes in their size, microscopic appearance and liberation of lactic acid from the films during the course of experiments demonstrated the association with GC release kinetics. These flexible films can be rolled into fibers with little change in release kinetics and the rolled MCAs can also be injected in vivo through a syringe needle to a delivery site. We envisage that this study could lead to an innovative approach to achieve prolonged release of GCs from biodegradable formulations at disease sites.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


