In Situ NADH-Activated BODIPY-Based Macrocyclic Supramolecular Photosensitizer for Chemo-Photodynamic Synergistic Tumor Therapy
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
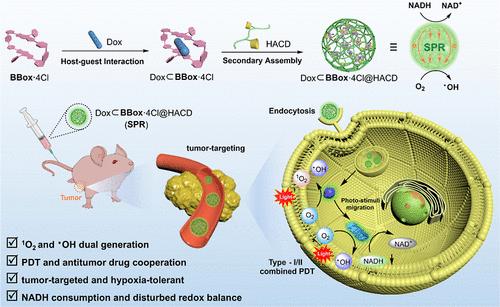
Photodynamic therapy (PDT) based on supramolecular assembly has been receiving wide attention due to its great potential application in clinical treatment. Herein, we report a supramolecular photoelectron “reservoir” (SPR) constructed by tetracationic boron dipyrromethene (BODIPY)-based macrocycle (BBox·4Cl), doxorubicin (Dox), and tumor-targeted β-cyclodextrin-grafted hyaluronic acid (HACD). Upon irradiation, BBox·4Cl can in situ catalyze nicotinamide adenine dinucleotide (NADH) to continuously generate electrons to inject into SPR, which further transfers electrons to oxygen, inducing highly efficient hydroxyl radical generation even under hypoxia. Synergistically, Dox in SPR as “pump” can be encapsulated by BBox·4Cl and transport photoelectrons between two BODIPY units, while HACD as “sponge” can enrich BBox·4Cl by the electrostatic interaction to concentrate them closer in space, which facilitates intramolecular and intermolecular photoelectron transfer, respectively, and significantly enhances the generation of hydroxyl radicals. Meanwhile, electron replenishment in SPR causes NADH depletion and redox dysfunction, thereby accelerating the apoptosis and achieving highly effective synergistic tumor therapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


