Design, Synthesis, and Biological Evaluation of Marchantin C-NO Donor Hybrids for Overcoming Pgp-Mediated Drug Resistance by Targeting Lysosome
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
A series of marchantin C-NO donor hybrids were designed, synthesized, and evaluated for their antitumor activity in vitro and in vivo. Notably, MC-furoxan hybrid 14 exhibited the best selective inhibitory activity against MCF-7/ADR (IC50 = 0.024 μM) with 883 times potency compared with MCF-7 cells (IC50 = 21.20 μM), and the cytotoxicity toward A549/Taxol (IC50 = 1.43 μM) increased 17-fold compared with that in A549 cells (IC50 = 23.75 μM). Preliminary pharmacological studies revealed that 14 could “hijack” the lysosomal Pgp and release NO to produce reactive oxygen species (ROS) in lysosomes, resulting in lysosomal membrane permeabilization (LMP) and potentiated cytotoxicity. Additionally, compound 14 achieved stronger antitumor activity and superior biosafety at relatively low doses than paclitaxel in the A549/Taxol xenograft model. In summary, this study provides a promising strategy for the design of such MC-furoxan hybrids like 14 to overcome MDR via the utilization of lysosomal Pgp transport activity.
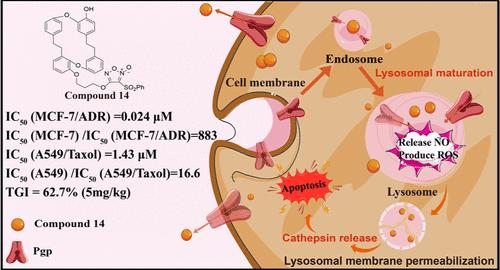
靶向溶酶体克服pgp介导的耐药的Marchantin C-NO供体杂种的设计、合成和生物学评价
设计、合成了一系列marchantin C-NO供体杂种,并对其体外和体内抗肿瘤活性进行了评价。其中,MC-furoxan杂种14对MCF-7/ADR (IC50 = 0.024 μM)的选择性抑制活性为MCF-7细胞(IC50 = 21.20 μM)的883倍,对A549/Taxol (IC50 = 1.43 μM)的细胞毒性为A549细胞(IC50 = 23.75 μM)的17倍。初步药理研究表明,14可以“劫持”溶酶体Pgp,释放NO,在溶酶体中产生活性氧(ROS),导致溶酶体膜渗透(LMP)和细胞毒性增强。此外,在A549/紫杉醇异种移植模型中,化合物14在相对低剂量下比紫杉醇具有更强的抗肿瘤活性和更好的生物安全性。总之,本研究为设计像14这样的mc -呋喃嘧啶杂交体提供了一种有希望的策略,通过利用溶酶体Pgp转运活性来克服MDR。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


