Anhydrous deep eutectic electrolyte: Enabling dendrite-free and highly stable zinc anodes
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
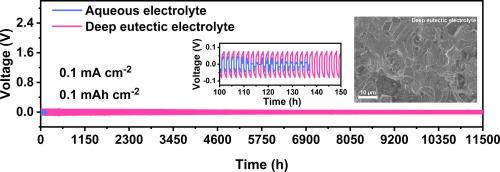
Zinc ion batteries (ZIBs) hold considerable theoretical promise due to their intrinsic advantages of cost-effectiveness and environmental sustainability. However, corrosion, hydrogen evolution reaction (HER) and dendrite problems associated with aqueous electrolytes severely limit the practical application of ZIBs. Here, we report a completely anhydrous deep eutectic electrolyte composed of choline chloride (ChCl), urea and ZnCl2, achieving dendrite-free growth and ultra-long cycling stability. ChCl, urea, and ZnCl2 are interconnected through hydrogen bonds to form the deep eutectic electrolyte, which broadens the electrochemical stability window (3.4 V vs. Ag/AgCl). In anhydrous deep eutectic electrolytes, the absence of water molecules results in the formation of a unique solvated structure of [ZnCl4(urea)]2−. The higher nucleation overpotential due to the complex intermolecular interactions favor the deposition of Zn2+ as small nuclei, which promotes compact Zn nucleation behavior and inhibits the formation of Zn dendrites. In addition, the urea protective layer adsorbed on the surface of the Zn anode provides effective corrosion protection. Benefiting from these advantages, the assembled Zn//Zn symmetric cell demonstrates exceptional cycle stability of 11500 h. The Zn||AC hybrid capacitor with the anhydrous deep eutectic electrolyte displays remarkable stability of 8000 cycles, achieving high power and energy densities of 84.0 W kg−1 at 34.1 Wh kg−1. Additionally, it is noteworthy that the cells using the eutectic electrolyte demonstrate excellent performance at 50 °C.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


