Orbital hybridization in graphene-based artificial atoms
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
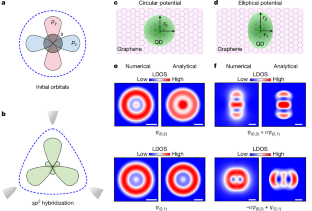
Intra-atomic orbital hybridization and interatomic bond formation are the two fundamental processes when real atoms are condensed to form matter1,2. Artificial atoms mimic real atoms by demonstrating discrete energy levels attributable to quantum confinement3–8. As such, they offer a solid-state analogue for simulating intra-atomic orbital hybridization and interatomic bond formation. Signatures of interatomic bond formation have been extensively observed in various artificial atoms9–17. However, direct evidence of the intra-atomic orbital hybridization in the artificial atoms remains to be experimentally demonstrated. Here we realize the orbital hybridization in artificial atoms by altering the shape of the artificial atoms. The anisotropy of the confining potential gives rise to the hybridization between quasibound states with different orbital quantum numbers within the artificial atom. These hybridized orbits are directly visualized in real space in our experiment and are well reproduced by both numerical calculations and analytical derivations. Our study opens an avenue for designing artificial matter that cannot be accessed on real atoms through experiments. Moreover, the results obtained inspire the progressive control of quantum states in diverse systems. Orbital hybridization in artificial atoms is achieved by altering their shape, and the anisotropy of the confining potential gives rise to the hybridization between quasibound states with different orbital quantum numbers within the artificial atom.

石墨烯人造原子的轨道杂化
原子内轨道杂化和原子间键形成是真实原子凝聚形成物质的两个基本过程。人造原子通过展示可归因于量子限制的离散能级来模拟真实原子3,4,5,6,7,8。因此,它们为模拟原子内轨道杂化和原子间键形成提供了固态模拟。在各种人造原子9,10,11,12,13,14,15,16,17中广泛观察到原子间键形成的特征。然而,人工原子中原子内轨道杂化的直接证据仍有待实验证明。这里我们通过改变人造原子的形状来实现人造原子的轨道杂化。约束势的各向异性引起了人造原子内具有不同轨道量子数的准束缚态之间的杂化。在我们的实验中,这些混合轨道在实际空间中直接可视化,并且通过数值计算和解析推导都可以很好地再现。我们的研究为设计无法通过实验在真实原子上获得的人造物质开辟了一条途径。此外,所得结果启发了不同系统中量子态的渐进控制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


