The endogenous antigen-specific CD8+ T cell repertoire is composed of unbiased and biased clonotypes with differential fate commitments
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Generating balanced populations of CD8+ effector and memory T cells is necessary for immediate and durable immunity to infections and cancer. Yet, a definitive understanding of how a diverse CD8+ T cell repertoire differentiates remains unclear. We identified several hundred T cell receptor (TCR) clonotypes that constitute the polyclonal response against a single antigen and found that a majority of TCR clonotypes were highly biased toward memory or effector fates. TCR-intrinsic biases were not stochastic and were dominant over environmental cues. Differential gene expression analysis of memory- or effector-biased TCR clonotypes showed bifurcation of differential fates at the early effector stage. Additionally, phylogenetic analysis revealed that memory-biased clonotypes retain their fate preferences in subclonal populations but effector-biased subclones can switch to a memory fate. Our study highlights that the polyclonal CD8+ T cell response is a composite of unbiased and biased clonotypes with varying capacity to incorporate environmental cues in their cell fate decisions.
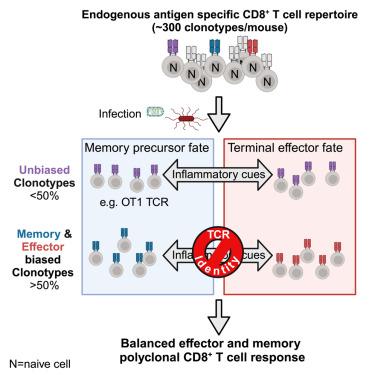
内源性抗原特异性CD8+ T细胞库由具有不同命运承诺的无偏和偏克隆型组成
产生平衡的CD8+效应T细胞和记忆T细胞群是对感染和癌症的即时和持久免疫所必需的。然而,对不同的CD8+ T细胞库如何分化的明确理解仍不清楚。我们鉴定了数百种T细胞受体(TCR)克隆型,这些克隆型构成了针对单一抗原的多克隆反应,并发现大多数TCR克隆型高度偏向于记忆或效应命运。tcr -内在偏差不是随机的,比环境因素更重要。记忆偏倚或效应偏倚的TCR克隆型的差异基因表达分析显示,在效应早期阶段,差异命运发生了分化。此外,系统发育分析显示,记忆偏倚的克隆型在亚克隆群体中保留了它们的命运偏好,但效应偏倚的亚克隆可以切换到记忆命运。我们的研究强调,多克隆CD8+ T细胞反应是无偏和偏克隆型的组合,具有不同的能力,将环境线索纳入其细胞命运决定中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


