Recent Progress in Radiosensitive Nanomaterials for Radiotherapy-Triggered Drug Release
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Benefiting from the unique properties of ionizing radiation, such as high tissue penetration, spatiotemporal resolution, and clinical relevance compared with other external stimuli, radiotherapy-induced drug release strategies are showing great promise in developing effective and personalized cancer treatments. However, the requirement of high doses of X-ray irradiation to break chemical bonds for drug release limits the application of radiotherapy-induced prodrug activation in clinics. Recent advances in nanomaterials offer a promising approach for radiotherapy sensitization as well as integrating multiple modalities for improved therapy outcomes. In particular, the catalytic radiosensitization that utilizes electrons and energy generated by nanomaterials upon X-ray irradiation has demonstrated excellent potential for enhanced radiotherapy. In this Review, we summarize the design principles of X-ray-responsive chemical bonds for controlled drug release, strategies for catalytic radiosensitization, and recent progress of X-ray-responsive nanoradiosensitizers for enhanced radiotherapy by integration with chemotherapy, chemodynamic therapy, photodynamic therapy, photothermal therapy, gas therapy, and immunotherapy. Finally, we discuss the challenges of X-ray-responsive nanoradiosensitizers heading toward possible clinical translation. We expect that emerging strategies based on radiotherapy-triggered drug release will facilitate a frontier in accurate and effective cancer therapy in the near future.
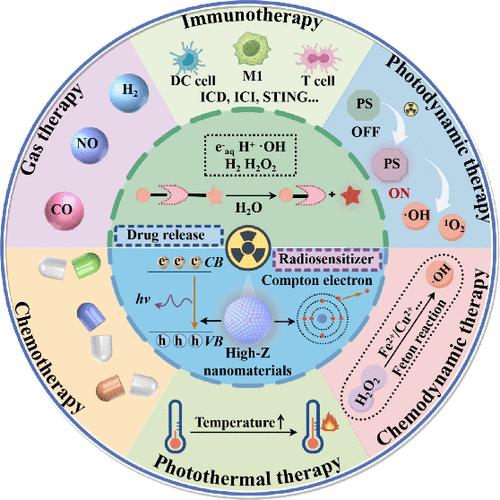
与其他外部刺激相比,电离辐射具有高组织穿透性、时空分辨率和临床相关性等独特特性,因此,放疗诱导药物释放策略在开发有效的个性化癌症治疗方法方面大有可为。然而,由于需要高剂量的 X 射线照射才能打破化学键使药物释放,这限制了放疗诱导原药活化在临床上的应用。纳米材料的最新进展为放射治疗增敏以及整合多种模式以改善治疗效果提供了一种前景广阔的方法。特别是,利用纳米材料在 X 射线照射下产生的电子和能量进行催化放射增敏,已显示出增强放射治疗的巨大潜力。在本综述中,我们总结了用于药物控释的 X 射线响应化学键的设计原理、催化放射增敏的策略,以及 X 射线响应纳米放射增敏剂与化疗、化学动力疗法、光动力疗法、光热疗法、气体疗法和免疫疗法相结合用于增强放疗的最新进展。最后,我们讨论了 X 射线响应纳米放射增敏剂走向临床应用所面临的挑战。我们期待,在不久的将来,基于放疗触发药物释放的新兴策略将推动准确有效的癌症治疗向前发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


