Inhibiting Neutrophil Extracellular Trap Formation through Iron Regulation for Enhanced Cancer Immunotherapy
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
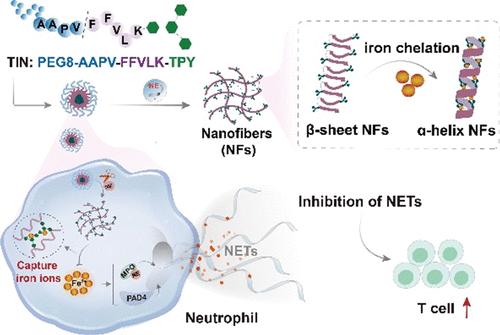
Iron metabolism of neutrophils plays a vital role in neutrophil extracellular trap (NET) formation, which presents as one of the major hurdles to the immune response in the tumor microenvironment. Here, we developed a peptide–drug conjugate (PDC)-based transformable iron nanochelator (TIN) equipped with the ability to regulate the iron metabolism of neutrophils, endowing inhibition of NET formation and the ensuing immunosuppression functions. The TIN could expose the iron-binding motifs through neutrophil elastase-mediated morphological transformation from nanoparticles to β-sheet nanofibers, which further evolve into stable α-helix nanofibers after chelation with iron(II) ions. This process enables a highly specific regulation of iron(II) ions of neutrophils, which turns into an efficient way of inhibiting NET formation and improving the immune response. Furthermore, the TIN showed an improved therapeutic effect in combination with protein arginine deiminase 4 inhibitors and synergistically boosted the anti-PD-L1 treatment. This study designates an iron-regulation strategy to inhibit NET formation, which provides an alternative approach to immune modulation from the perspective of targeting the iron metabolism of neutrophils in cancer immunotherapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


