Stabilizing Li-Metal Electrode via Anion-Induced Desolvation in a Covalent Organic Framework Separator
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
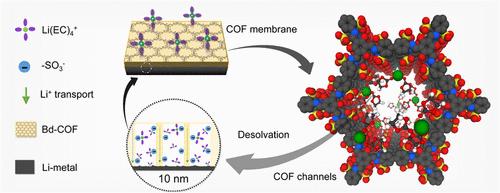
Although Li-metal batteries have been widely used as high-capacity batteries, they are highly susceptible to electrolytes that lead to dendritic or dead Li growth, which significantly reduces the stability of Li-metal electrodes. Herein, we report an anionic covalent organic framework (sulfonate COF: Bd-COF) as a Li+-solvate dissociator that strips solvent molecules from encapsulated Li+ to stabilize Li-metal electrodes. The homogeneous and dense ionic COF separator was prepared using a template-assisted interface in-suit polymerization engineering. Notably, the well-developed anionic groups within the COF channels could as counter-charge ligands to Li+, that adsorb Li+-solvates and induce their partial desolvation. Meanwhile, the ordered anionic groups on the surface of COF pores provide continuous ion channels for Li+ migration, facilitating the removal of solvent molecules from Li+-solvated species. Combined with the dense nanoporous feature, the COF membrane was found to be effective in suppressing Li-dendrites and parasitic reactions. The Bd-COF/Celgard membrane realizes uniform Li deposition on Li-metal electrodes, exhibiting excellent cycling performance in Li-symmetric batteries and high-voltage Li-metal batteries with LiNi0.6Mn0.2Co0.2O2 cathodes, showcasing the application prospects of ion-conductive covalent organic frameworks in lithium battery separators.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


