Heterogenous HER activity of Ni(II)N2S2 molecular catalysts
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
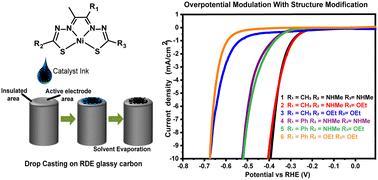
Green hydrogen, generated through the electrolysis of water using renewable energy sources, is recognized as a highly promising alternative to fossil fuels in the pursuit of net-zero carbon emissions. Electrocatalysts are crucial for reducing overpotentials and enhancing the efficiency of the hydrogen evolution reaction (HER) for the production of green hydrogen. Homogeneous HER serves as a primary method to assess the activity and mechanisms of novel non-precious molecular electrocatalysts in pursuit of replacing precious platinum standards. However, these catalysts can sometimes exhibit instability under reductive and acidic conditions during homogeneous HER. Thus, it is also essential to evaluate catalysts through heterogeneous HER for initial assessment and practical application. In this study, we examine a series of structurally related N2S2 chelated Ni(II) complexes, which are tailored to optimize the basicity of the catalyst for heterogeneous HER activity. These complexes are insoluble in 0.5 M H2SO4, and the films formed after catalyst deposition on glassy carbon electrodes (GCEs) exhibit catalytic currents during HER, demonstrating moderate to good overpotentials, Tafel slopes, and charge transfer resistance. Furthermore, we observe the anticipated structure–activity relationship that arises from tuning the catalyst structure. The complexes maintain stability over extended reductive cycling, as confirmed by various surface characterization techniques, including SEM, EDX, XPS, and XRD. This study highlights the potential of utilizing catalyst basicity to develop efficient and robust heterogeneous HER catalysts.
Ni(II)N2S2 分子催化剂的异源 HER 活性
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


