Chemical Synthesis, Refolding, and Characterization of Mirror-Image Cyclophilin A
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
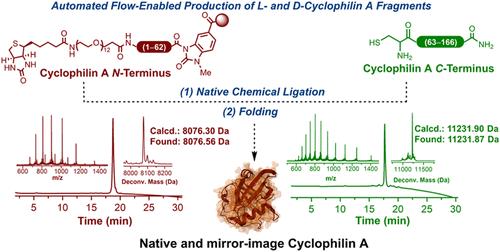
The chemical synthesis of proteins (CSP) has been an essential tool in studying and understanding the role of these biological polymers and in enabling the discovery of novel classes of inhibitors. However, CSP with commercially available synthesizers is typically limited to producing polypeptides of about 50 to 70 amino acids in length. Consequently, a wide range of protein targets have been inaccessible using these technologies, or they require cumbersome synthesis and purification of multiple peptide fragments. In this report, we employed a powerful combination of automated fast-flow peptide synthesis (AFPS), native chemical ligation (NCL), and high-throughput evaluation of refolding conditions to achieve the first chemical synthesis of both the wild-type and mirror-image forms of functional full-length cyclophilin A, which plays a vital role in proline cis–trans isomerization and other important processes. Functional assays confirmed that the chemically synthesized proteins retained their biological properties.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


