Electrochemical 1,2-Alkylarylation of Styrenes with Malonates and N-Heteroarenes via Direct C(sp3)–H/C(sp2)–H Functionalization
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A general and atom-economical electrochemical dehydrogenative method for the intermolecular 1,2-alkylarylation of styrenes with malonates and nucleophilic N-heteroarenes (including indoles and pyrroles) has been developed. This transformation provides a regioselective route to construct highly valuable 1,1-diarylalkanes enabled by C(sp3)–H/C(sp2)–H functionalization under mild conditions, and H2 is the only theoretical byproduct. Mechanistic studies indicated that the reaction proceeded through the oxidative of the C(sp3)–H bond to generate alkyl radical, radical addition across the C═C bond, single electron oxidation and C(sp2)–H functionalization cascades.
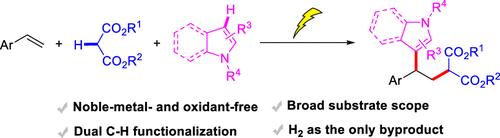
苯乙烯与丙二酸酯和n -杂芳烃直接C(sp3) -H /C(sp2) -H功能化的1,2-烷基化反应
提出了苯乙烯与丙二酸酯和亲核n -杂芳烃(包括吲哚和吡咯)分子间1,2-烷基化反应的通用原子经济电化学脱氢方法。这种转化为在温和条件下通过C(sp3) -H /C(sp2) -H功能化构建高价值的1,1-二芳烷提供了区域选择性途径,H2是理论上唯一的副产物。机理研究表明,该反应通过C(sp3) -H键氧化生成烷基自由基、C = C键上的自由基加成、单电子氧化和C(sp2) -H功能化级联反应进行。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


