Nonmelting Disordering Facilitated by Electron Delocalization
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
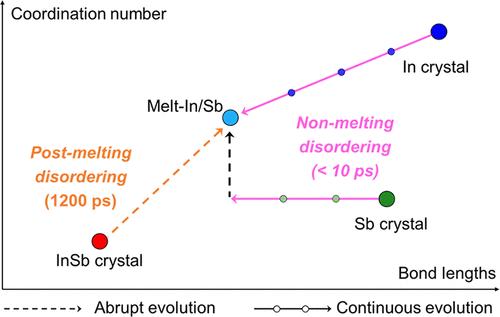
Disordering atomic structures offers a functionality hardly expected in ordered states, including phase-change memory and photonic computing, offering the potential to renovate von Neumann architecture for neuromorphic engineering with low latency. However, significant energy consumption during the disordering compromises the data reliability and integration efficiency, which is traditionally regarded to take place after melting. Here, we investigate time for disordering in isochronal and isochoric manners, challenging the conventional melt-quenching theory. The disordering times of pure Sb, Ag–In–Sb–Te, and In surpass that of InSb by over 50 times, despite a higher melting point and a lower laser absorption rate of Sb compared to InSb. This nontrivial contrast is elucidated by theoretical calculation that delocalized electrons enable flexible modification of bond lengths even below the melting points where undermined bond directionality provides room for atoms to depart from their original positions. Facilitated by delocalized electrons, specifically through metavalent and metallic bonding rather than covalent bonding, atoms can be disordered without undergoing melting, which aligns with the rapid disordering of Sb compared to that of InSb. The results bridge the unaddressed gap between chemical interaction and kinetic behaviors during the disordering and suggest design rules highlighting electron-delocalization rather than solely relying on melting points to improve energy efficiency.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


