Stable crystalline keteniminyl anions
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
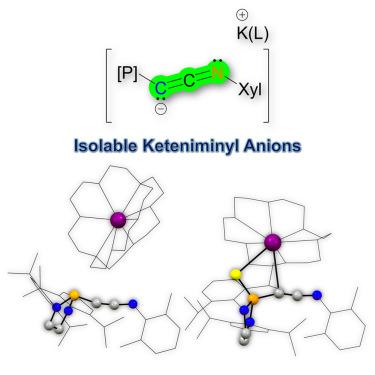
The synthesis, characterization, and reactivity of keteniminyl anions [R1C=C=NR2]− (R1 = diazaphospholidinyl or diazathiophospholidinyl, R2 = 2,6-dimethylphenyl), a hitherto uncharted functional group, are the primary focus of this study. The keteniminyl anions are characterized by their nucleophilic/basic anionic carbon and π electrons, which are extensively delocalized along the PCCN chain. These anions undergo a range of facile reactions, such as protonation, alkylation, silylation, and metalation at the carbon site, leading to various ketenimine derivatives. They also participate in hydroamination reactions, yielding amino enamide functional groups. Additionally, the diazaphospholidinyl substituent in keteniminyl anions acts as a previously underappreciated weak π-electron acceptor when the phosphorus atom is in a pyramidalized state, thereby facilitating the stabilization of the electron-rich anionic carbon. The isolation of such keteniminyl anions not only represents a significant synthetic achievement but also heralds the potential for the future isolation of electron-rich species featuring diazaphospholidinyl substituents.
稳定结晶氯胺基阴离子
酮亚胺基阴离子[R1C=C=NR2]−(R1 = diazaphospholinyl或diazathiophospholinyl, R2 = 2,6-二甲基苯基)的合成、表征和反应性是本研究的主要重点,这是一个迄今为止未知的官能团。酮亚胺基阴离子的特征是它们的亲核/碱性阴离子碳和π电子,它们沿着PCCN链广泛地离域。这些阴离子在碳位上发生一系列容易发生的反应,如质子化、烷基化、硅基化和金属化,从而产生各种酮亚胺衍生物。它们也参与氢胺化反应,产生氨基酰胺官能团。此外,当磷原子处于金字塔化状态时,二氮磷基取代基在酮亚胺基阴离子中充当弱π电子受体,从而促进了富电子阴离子碳的稳定。这种乙烯基阴离子的分离不仅代表了一项重大的合成成就,而且预示着未来分离具有重氮磷基取代基的富电子物种的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


