Integration of a non-precious pyrolyzed Cu-doped ZIF as an oxygen depolarized cathode in an advanced chlor-alkali electrolyzer
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
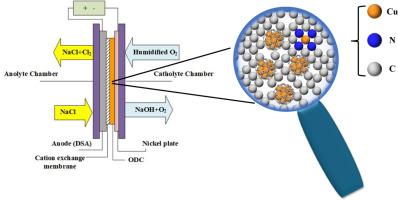
Oxygen reduction is the critical step in advanced chlor-alkali electrolysis, which has motivated extensive research in catalyst development for improved efficiency and durability. This study investigates the oxygen reduction reaction (ORR) on Cu-based electrocatalysts supported on N-doped carbon (Cu/NC), derived from a Cu-modified zeolitic imidazolate framework (ZIF), and their ultimate performance in a chlor-alkali electrolyzer. Through comprehensive electrochemical characterization in 0.1 M NaOH solution, values of Eonset = 0.87 V and E1/2 = 0.75 V (vs. RHE) were obtained, which are competitive with commercial Pt/C despite the superior j achieved by the latter in LSV tests. The electron transfer number (n) of the optimum Cu/NC was 4, very close to benchmark catalyst Pt/C 20 wt. % (n = 3.94). Cu/NC had a low Tafel slope (128 mV dec−1), thus speeding up the ORR on this nanocatalyst. Additionally, chronoamperometry and accelerated durability tests demonstrated the long-term stability of Cu/NC for 10 h. The catalyst was assembled as an oxygen depolarized cathode (ODC) in a purpose-designed advanced chlor-alkali electrolyzer, resulting in a cell voltage of 2.1 V at 1 kA m-2 and 80 °C, which underscores the potential of Cu-based nanocatalysts in electrochemical energy devices. This research serves to leverage insights for the use of advanced electrocatalysts to enhance the efficiency and sustainability of chlor-alkali electrolysis.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


