Electrochemical Generation of Hydrogen Peroxide Using Cerium Oxide Nanostructures Supported on Graphene: Synthesis, Characterization, and Application in Wastewater Treatment
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
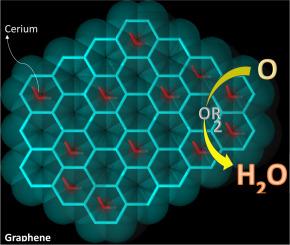
This study investigates the electrochemical generation of hydrogen peroxide (H₂O₂) using three distinct cerium oxide nanostructures—nanorods, nanospheres, and nanoparticles—supported on graphene. The nanorods and nanospheres were synthesized via the hydrothermal method, with controlled adjustments to centrifugation speed, calcination temperature, and duration to tailor their morphology. Cerium nanoparticles were prepared using a coprecipitation method. These nanostructures were employed to modify graphene and were evaluated for their performance in H₂O₂ electrogeneration. Among them, cerium nanorods exhibited the highest H₂O₂ production, achieving a 91% efficiency. This superior performance can be attributed to the presence of oxidizing species (Ce³⁺), as identified through Raman spectroscopy, along with oxygen vacancies and enhanced surface hydrophilicity. Scanning electron microscopy (SEM) revealed that the nanorods were optimally distributed below the graphene sheets. The hydrothermal and coprecipitation synthesis methods proved effective for producing cerium oxide nanostructures with high catalytic activity. These findings highlight the potential of cerium oxide nanostructures as efficient catalysts for wastewater treatment via the electro-Fenton process.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


