Integrating voltammetry in ecotoxicology: Cu(II)-nitrocatechol complexes formation as a driver of Cu(II) and nitrocatechol toxicity in aquatic systems
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Nitrocatechols (NCs) are hazardous environmental pollutants that pose a significant risk to aquatic organisms. The toxicity of pollutants is determined by their speciation, which depends on interactions with coexisting inorganic and organic substances, emphasizing the need for detailed specific studies. Employing square wave and cyclic voltammetry, we investigated the complexation between NCs, specifically 4-nitrocatechol (4NC), 3-methyl-4-nitrocatechol (3M4NC), 3-methyl-5-nitrocatechol (3M5NC), and 4-methyl-5-nitrocatechol (4M5NC), and copper ions (Cu(II)), a micronutrient that is toxic at elevated concentrations. By confirming and following a quasi-reversible two-electron reduction of di-nitrocatecholate [Cu(NC)2]2- complexes in slightly alkaline solution (pH 8.2), we demonstrated fast complex formation in solution at different analyte concentrations and high kinetic stability. However, the pH lowering resulted in degradation of these complexes, yielding mono-nitrocatecholate [Cu(NC)] species and eventually uncomplexed Cu(II) ions. The chronic toxicity bioassays (AlgaeTox) with Scenedesmus subspicatus microalgae exposed to a mixture of Cu(II) and NCs at different concentrations showed decreased toxicity compared to the toxicity of individual Cu(II) and NCs. This decrease was linked to the formation of [Cu(NC)2]2− complexes, which was confirmed by the appearance of a corresponding voltammetric reduction peak. Conversely, increased toxicity was observed when the complexes were degraded, resulting in the presence of free/labile Cu(II) or NCs species in the solution. These results demonstrate that concentration-dependent complexation between pollutants significantly alters the toxicity profile of Cu(II)/NC systems. This study highlights the value of voltammetry with a mercury drop working electrode as a reliable method for studying pollutant complexation relevant to ecotoxicity in natural waters.
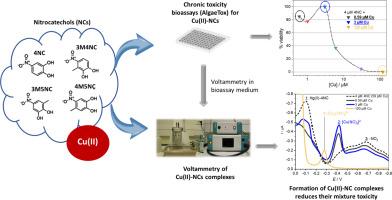
二硝基邻苯二酚(NCs)是一种有害的环境污染物,对水生生物构成重大风险。污染物的毒性由其种类决定,而种类又取决于与共存的无机和有机物质的相互作用,因此需要进行详细的具体研究。我们采用方波伏安法和循环伏安法研究了 NC(特别是 4-硝基-4-儿茶酚 (4NC)、3-甲基-4-硝基儿茶酚 (3M4NC)、3-甲基-5-硝基儿茶酚 (3M5NC) 和 4-甲基-5-硝基儿茶酚 (4M5NC))与铜离子(Cu(II))(一种在浓度升高时具有毒性的微量元素)之间的络合作用。通过在微碱性溶液(pH 值为 8.2)中确认和跟踪二硝基儿茶酚[Cu(NC)2]2- 复合物的准可逆双电子还原过程,我们证明了在不同分析物浓度下溶液中复合物的快速形成和高度的动力学稳定性。然而,pH 值降低会导致这些络合物降解,产生单硝基邻苯二甲酸盐[Cu(NC)]物种,最终产生未络合的 Cu(II) 离子。与单个 Cu(II)和 NCs 的毒性相比,Scenedesmus subspicatus 微藻暴露于不同浓度的 Cu(II) 和 NCs 混合物的慢性毒性生物测定(AlgaeTox)显示毒性有所降低。这种毒性降低与[Cu(NC)2]2- 复合物的形成有关,相应伏安还原峰的出现证实了这一点。相反,当络合物被降解,导致溶液中存在游离/失效的 Cu(II) 或 NCs 物种时,毒性就会增加。这些结果表明,污染物之间随浓度变化的络合作用极大地改变了 Cu(II)/NC 系统的毒性特征。这项研究强调了汞滴工作电极伏安法作为研究与自然水体生态毒性相关的污染物络合的可靠方法的价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


