Hepatic sphingomyelin phosphodiesterase 3 promotes steatohepatitis by disrupting membrane sphingolipid metabolism
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Metabolic-dysfunction-associated steatohepatitis (MASH) remains a major health challenge. Herein, we identify sphingomyelin phosphodiesterase 3 (SMPD3) as a key driver of hepatic ceramide accumulation through increasing sphingomyelin hydrolysis at the cell membrane. Hepatocyte-specific Smpd3 gene disruption or pharmacological inhibition of SMPD3 alleviates MASH, whereas reintroducing SMPD3 reverses the resolution of MASH. Although healthy livers express low-level SMPD3, lipotoxicity-induced DNA damage suppresses sirtuin 1 (SIRT1), triggering an upregulation of SMPD3 during MASH. This disrupts membrane sphingomyelin-ceramide balance and promotes disease progression by enhancing caveolae-dependent lipid uptake and extracellular vesicle secretion from steatotic hepatocytes to exacerbate inflammation and fibrosis. Consequently, SMPD3 acts as a central hub integrating key MASH hallmarks. Notably, we discovered a bifunctional agent that simultaneously activates SIRT1 and inhibits SMPD3, which shows significant therapeutic potential in MASH treatment. These findings suggest that inhibition of hepatic SMPD3 restores membrane sphingolipid metabolism and holds great promise for developing novel MASH therapies.
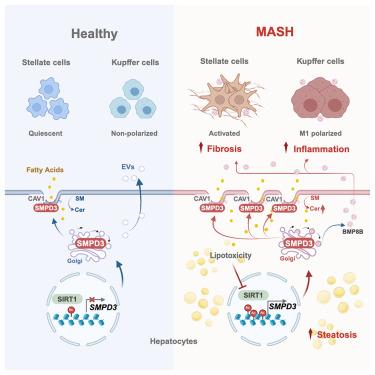
肝鞘磷脂磷酸二酯酶3通过破坏鞘脂膜代谢促进脂肪性肝炎
代谢功能障碍相关的脂肪性肝炎(MASH)仍然是一个主要的健康挑战。在此,我们发现鞘磷脂磷酸二酯酶3 (SMPD3)通过增加细胞膜鞘磷脂水解而成为肝神经酰胺积累的关键驱动因素。肝细胞特异性Smpd3基因破坏或药理抑制Smpd3可缓解MASH,而重新引入Smpd3可逆转MASH的消退。虽然健康的肝脏表达低水平的SMPD3,但脂肪毒性诱导的DNA损伤抑制sirtuin 1 (SIRT1),在MASH期间触发SMPD3的上调。这会破坏鞘磷脂-神经酰胺膜平衡,并通过增强脂肪变性肝细胞的小泡依赖性脂质摄取和细胞外囊泡分泌,从而加剧炎症和纤维化,从而促进疾病进展。因此,SMPD3充当集成关键MASH标志的中心集线器。值得注意的是,我们发现了一种同时激活SIRT1和抑制SMPD3的双功能药物,在MASH治疗中显示出显著的治疗潜力。这些发现表明,抑制肝脏SMPD3可以恢复鞘脂膜代谢,并有望开发新的MASH治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


