An mRNA vaccine encoding proteasome-targeted antigen enhances CD8+ T cell immunity
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
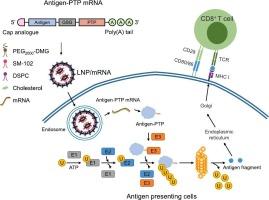
The efficient induction of antigen-specific CD8+ T cell activation is crucial in the development of mRNA tumor vaccines. Endogenous antigens are primarily degraded through the ubiquitin-proteasome system, followed by antigen presentation via major histocompatibility complex class I (MHC-I) molecules, leading to the activation of CD8+ T cells. Therefore, in this study, a novel mRNA vaccine was developed by fusing the mRNA sequence encoding the antigen with a proteasome-targeting peptide (PTP), aiming to enhance proteasomal targeting of the antigen and facilitate its degradation through the ubiquitin-proteasome system, thereby inducing a stronger CD8+ T cell immune response. This study confirmed a significant increase in antigen expression of the antigen-PTP fused mRNA vaccine upon treatment with a VHL inhibitor, as well as notable upregulation of genes associated with the MHC-I antigen-presenting pathway following treatment with the antigen-PTP fused mRNA vaccine. The intramuscular administration of the antigen-PTP fused mRNA vaccine significantly promoted the activation of dendritic cells, macrophages, and T cells in draining lymph nodes and spleens. Additionally, in TC-1 tumor-bearing mice, it markedly suppressed tumor growth, facilitated infiltration of intratumoral antigen-specific CD8+ T cells, and induced immune memory.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


