Photocatalytic CO2 reduction catalysed by 3d transition metal complexes bearing an S2N2 ancillary ligand equipped with pyridine pendants as binding sites for Lewis acids
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
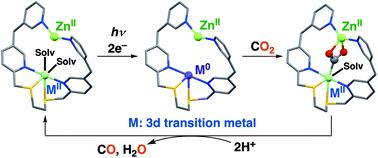
To solve the current environmental and energy problems, the development of an efficient photocatalytic system for CO2 reduction, producing useful chemical resources such as CO, is a promising approach. Herein, we have synthesized 3d-transition metal complexes (Mn, Fe, and Co) using an S2N2-type ligand (1), inspired by the structure of a natural CO2-fixing enzyme, [NiFe]CODH, as CO2-reducing catalysts. When a Zn2+ salt was added as a Lewis acid to a solution of one of the complexes, containing [Ru(bpy)3]2+ as a photosensitizer and BIH (= 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole) as a sacrificial reductant in dimethylacetamide/H2O (9 : 1, v/v), the amount of CO evolved under photoirradiation at 450 nm was increased in comparison with that without the Zn2+ salt. Notably, the selectivity of CO formation by 1-Co was enhanced from 73% to 98% under photoirradiation at 450 nm, and the TON for CO formation by 1-Mn increased to 875 after irradiation for 4 h.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


