Isomeric orientation of the S atom in thiophene of benzodithiophene-4,8-dione to achieve a high-performance electrochromic polymer†
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
In this work, two pairs of D–A–D type isomeric monomers, ETTD, EBDD and EETTD, EEBDD, were designed and synthesized by modifying the orientation of the thiophene ring and the conjugation length of the molecular backbone based on benzodithiophene-4,8-dione acceptor units. We also successfully synthesized polymers PEETTD and PEEBDD via electrochemical polymerization, but failed to obtain PETTD and PEBDD from ETTD and EBDD. These subtle structural changes significantly impacted the optical and electrochemical properties and polymerization of the monomers. Notably, EEBDD exhibited the lowest onset oxidation potential (0.44 V) and the strongest absorption at 486 nm, while EETTD displayed the most red-shifted absorption edge. PEEBDD demonstrated impressive electrochromic properties, including a better optical contrast of 39.9% and a coloration efficiency of 320.7 cm2 C−1 at 660 nm when compared to PEETTD. These results reveal the importance of thiophene ring orientation and conjugation length in tuning the electrochromic performance of polymers, offering a promising approach for designing high-performance electrochromic materials.

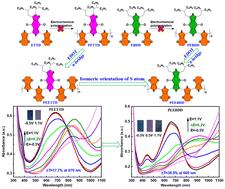
苯并二噻吩-4,8-二酮噻吩中 S 原子的异构取向实现高性能电致变色聚合物
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


