Synthesis and Luminescent Behavior of Lead, Manganese, and Copper Containing Organic–Inorganic Metal Halides
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Organic–inorganic metal halides (OIMHs) have attracted considerable attention owing to their unique optoelectronic properties. Here, we successfully synthesized five lead, manganese and copper containing OIMHs based on 4-aminodiphenylmethane (C13H13N) and 4,4′-methylenedianiline (C13H14N2). Among them, (C13H14N)2PbCl4 and (C13H14N)2PbBr4, both exhibiting two-dimensional frameworks, crystallize into the Pmm2 and P21/c space groups, respectively, and present optical band gaps of approximately 3.6 and 3.0 eV. (C13H14N)2PbCl4 demonstrates blue light emission centered at 502 nm with a substantial full width at half-maximum (fwhm) of 180 nm and a Stokes shift of 182 nm, originating from self-trapped exciton emission. In contrast, (C13H14N)2PbBr4 displays blue photoluminescence characterized by a fwhm of 26 nm and an emission peak at 417 nm, corresponding to free exciton emission. Additionally, we investigated three zero-dimensional OIMHs: (C13H14N)4MnCl6·Cl, (C13H15N2)4CuCl10·4H2O, and [(C13H16N2)(C2H6NH2)MnCl4·2H2O]·Cl. Our research delves into their fundamental optical properties and luminescence mechanisms, providing valuable insights for future studies.
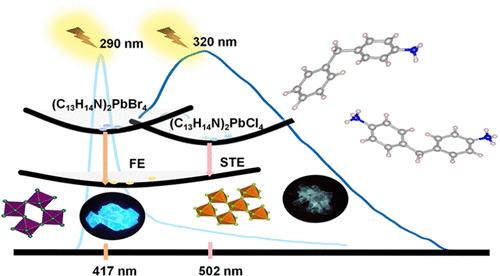
含有机-无机金属卤化物的铅、锰、铜的合成及其发光行为
有机-无机金属卤化物(OIMHs)因其独特的光电性能而受到广泛关注。本文以4-氨基二苯基甲烷(C13H13N)和4,4′-亚甲苯胺(C13H14N2)为原料,成功合成了5种含铅、锰和铜的OIMHs。其中(C13H14N)2PbCl4和(C13H14N)2PbBr4均为二维骨架,分别结晶为Pmm2和P21/c空间基团,光学带隙约为3.6 eV和3.0 eV。(C13H14N)2PbCl4显示出以502nm为中心的蓝光发射,半峰全宽为180nm, Stokes位移为182nm,这是由自捕获激子发射引起的。相比之下,(C13H14N)2PbBr4表现出蓝色光致发光,其峰宽为26 nm,发射峰位于417 nm,对应于自由激子发射。此外,我们还研究了三种零维OIMHs: (C13H14N)4MnCl6·Cl, (C13H15N2)4CuCl10·4H2O和[(C13H16N2)(C2H6NH2)MnCl4·2H2O]·Cl。我们的研究深入研究了它们的基本光学性质和发光机制,为未来的研究提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


