Programs, origins and immunomodulatory functions of myeloid cells in glioma
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
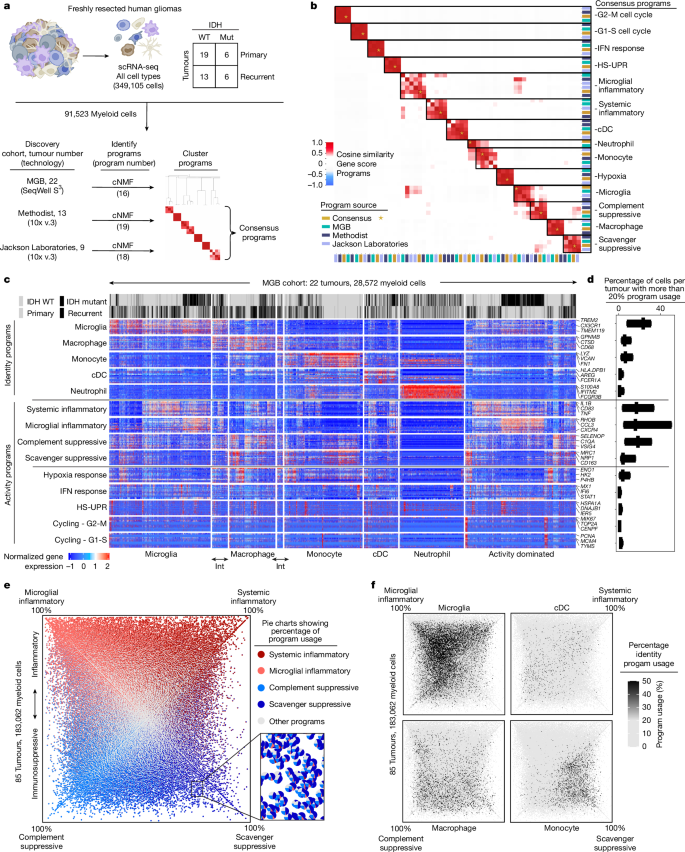
Gliomas are incurable malignancies notable for having an immunosuppressive microenvironment with abundant myeloid cells, the immunomodulatory phenotypes of which remain poorly defined1. Here we systematically investigate these phenotypes by integrating single-cell RNA sequencing, chromatin accessibility, spatial transcriptomics and glioma organoid explant systems. We discovered four immunomodulatory expression programs: microglial inflammatory and scavenger immunosuppressive programs, which are both unique to primary brain tumours, and systemic inflammatory and complement immunosuppressive programs, which are also expressed by non-brain tumours. The programs are not contingent on myeloid cell type, developmental origin or tumour mutational state, but instead are driven by microenvironmental cues, including tumour hypoxia, interleukin-1β, TGFβ and standard-of-care dexamethasone treatment. Their relative expression can predict immunotherapy response and overall survival. By associating the respective programs with mediating genomic elements, transcription factors and signalling pathways, we uncover strategies for manipulating myeloid-cell phenotypes. Our study provides a framework to understand immunomodulation by myeloid cells in glioma and a foundation for the development of more-effective immunotherapies. A study of myeloid cells in gliomas, a type of brain tumour, used a factor-based computational framework to reveal four immunomodulatory gene-expression programs that are expressed across myeloid cell types, driven by microenvironmental cues and predictive of therapeutic response.

骨髓细胞在胶质瘤中的程序、起源和免疫调节功能
胶质瘤是一种无法治愈的恶性肿瘤,其显著特征是具有免疫抑制微环境和丰富的骨髓细胞,其免疫调节表型仍不明确1。在这里,我们通过整合单细胞RNA测序,染色质可及性,空间转录组学和胶质瘤类器官外植体系统系统地研究这些表型。我们发现了四种免疫调节表达程序:原发性脑肿瘤特有的小胶质细胞炎症和清道夫免疫抑制程序,以及非脑肿瘤也表达的全身炎症和补体免疫抑制程序。这些项目并不取决于骨髓细胞类型、发育起源或肿瘤突变状态,而是由微环境因素驱动,包括肿瘤缺氧、白细胞介素-1β、TGFβ和标准的地塞米松治疗。它们的相对表达可以预测免疫治疗反应和总生存期。通过将各自的程序与介导的基因组元件、转录因子和信号通路联系起来,我们发现了操纵髓细胞表型的策略。我们的研究为理解骨髓细胞在胶质瘤中的免疫调节提供了一个框架,并为开发更有效的免疫疗法奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


