New Synthesis and Pharmacological Evaluation of Enantiomerically Pure (R)- and (S)-Methadone Metabolites as N-Methyl-d-aspartate Receptor Antagonists
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
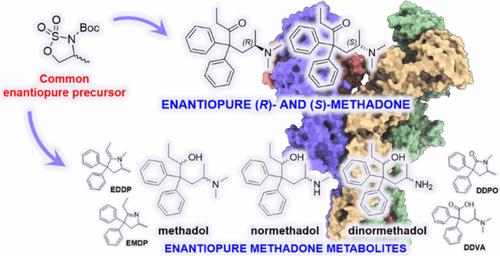
N-Methyl-d-aspartate receptor (NMDAR) is gaining increasing interest as a pharmacological target for the development of fast-acting antidepressants. (S)-Methadone (esmethadone), has recently shown promising efficacy for the treatment of major depressive disorder. However, methods for its enantiopure preparation still rely on complex and expensive resolution procedures. In addition, enantiopure methadone metabolites have never been evaluated for their NMDAR activity. Here, we report the development of a novel chiral pool approach, based on cyclic sulfamidate ring-opening reaction, for the asymmetric synthesis of (R)- and (S)-methadone, and the application of this methodology to the stereodivergent synthesis of 20 enantiopure methadone metabolites. The compounds were evaluated for their NMDAR antagonism and for their affinity toward a series of relevant CNS receptors. Strikingly, N-demethylated (6R)-methadol metabolites retain the higher NMDAR uncompetitive antagonism of (R)-methadone, while presenting lower opioid receptor affinity compared to (S)-methadone. These compounds could represent novel candidates for drug development in CNS disorders.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


