Chiral 3,3′-diaroyl BINOL phosphoric acids: syntheses and evaluation in asymmetric transfer hydrogenation, photophysical, and electrochemical studies†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
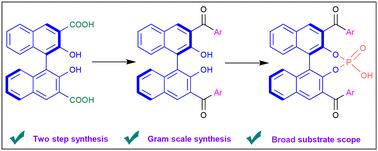
An efficient synthesis of enantiomerically pure 3,3′-diaroyl BINOLs is accomplished through chemoselective Weinreb ketone synthesis from the Weinreb amide derivative of chiral BINOL-3,3′-dicarboxylic acid using a Grignard reagent. This protocol facilitated the introduction of the aroyl group at the 3,3′-position of binaphthol. The 3,3′-diaroyl BINOL phosphoric acid has been prepared and evaluated for asymmetric transfer hydrogenation of 2-aryl/alkyl quinolines. The 3,3′-diaroyl BINOL phosphoric acids are found to be efficient catalysts in the hydrogenation of 2-aryl quinolines using Hantzsch ester to generate 2-aryl tetrahydroquinolines in excellent yields with moderate enantioselectivity. The presence of aroyl units, a photosensitizer core, in 3,3′-diaroyl BINOL prompted us to evaluate their photophysical and electrochemical properties, as these molecules may be a potential candidate for asymmetric photocatalysis.
手性3,3'-二芳基BINOL磷酸:不对称转移加氢的合成和评价,光物理和电化学研究。
手性binol -3,3'-二羧酸的Weinreb酰胺衍生物采用格氏试剂进行化学选择性Weinreb酮合成,实现了对映体纯3,3'-二羰基BINOLs的高效合成。该方案有助于在联萘酚的3,3'位置上引入芳基。制备了3,3′-二芳基BINOL磷酸,并对2-芳基/烷基喹啉的不对称转移加氢进行了评价。3,3′-二芳基BINOL磷酸是2-芳基喹啉加氢反应的有效催化剂,利用Hantzsch酯制备2-芳基四氢喹啉,收率高,对映选择性适中。在3,3'-二芳基BINOL中存在芳香基单元(光敏剂核心),这促使我们评估它们的光物理和电化学性质,因为这些分子可能是不对称光催化的潜在候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


