Redox-active tin(II) complexes with sterically demanding o-phenylenediamido ligands and their reactivity with organic azides
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
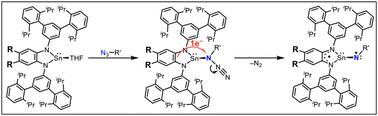
A series of tin(II) complexes R1 supported by phenylene-1,2-diamido ligands containing a bulky N-substituent TIPT (2,4,2′′,4′′-tetraisopropyl-[1,1′:3′,1′′]terphenyl) and different aromatic substituents R (Cl, H, Me, OMe) at the 4,5-positions and by a naphthalene-2,3-diamido ligand with the TIPT substituent naph1 are synthesised and characterised. Tin(II) complexes SnLMe and SnLPh(tBu)2 supported by phenylene-1,2-diamido ligands with sterically less hindered N-substituents, Ph or 3,5-di-tert-butylphenyl, are also prepared as reference complexes. Crystal structures of R1 and naph1 show that the tin(II) centers are coordinated with the two amido nitrogen atoms of the respective deprotonated chelating ligand and two solvent molecules such as tetrahydrofuran and/or acetonitrile. On the other hand, the tin(II) complex SnLPh(tBu)2 contains only one coordinating solvent molecule, and its tin(II) center exhibits intermolecular interaction with the aromatic ligand moiety of a neighboring tin(II) complex through a 5p–π interaction. The 119Sn NMR signal of R1 in C6D6 shifts from the lower- to higher-magnetic field region as R becomes more electron-withdrawing, which can be explained by assuming that the naked two-coordinate tin(II) complex and solvent-involving three- and/or four-coordinate tin(II) complex exist in equilibrium in solution. The tin(II) complexes with the sterically demanding ligands (R1 and naph1) are more stabilised against hydrolysis when compared with SnLPh and SnLPh(tBu)2. The tin(II) complexes R1 and naph1 undergo one-electron quasi-reversible ligand-based redox oxidation in a range from −0.45 V to +0.13 V vs. Fc/Fc+. The tin(II) complexes having electron-donating groups (R = OMe, Me, H) exhibit intramolecular C–H amination reactivity when treated with trisylazide (2,4,6-triisopropylphenylsulfonyl azide), giving sultam (5,7-diisopropyl-2,3-dihydro-3,3-dimethyl-1,2-benzothiazole-1,1-dioxide) as the product. On the other hand, the tin(II) complexes having electron-withdrawing groups (R = Cl, naph) do not show such reactivity. Such a difference in the reactivity is attributed to availability of a nitrene-radical bound species formed by the reaction. The formation step of the nitrene-radical bound species is analyzed kinetically to reveal that the reaction comprises two steps: binding of the organic azide to the tin(II) centre and successive intramolecular electron transfer from the ligand to the azide moiety to induce dinitrogen (N2) elimination and nitrene-radical formation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


