Scalable access to functional nylon 6 via ring-opening copolymerization of biobased δ-valerolactam with ε-caprolactam†
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
Abstract
Copolymerization of ε-caprolactam with functional lactam monomers was an effective strategy to introduce pendent substituents into nylon 6, which might endow these materials with improved processing properties, solubility, elasticity, and adhesion, while not compromising their inherent thermal and mechanical properties. However, the scalable synthesis of functional lactams remained extremely difficult due to their high raw material cost and/or cumbersome synthesis steps, making it difficult to meet the enormous market demand for nylon 6 materials. Herein, we introduced a new biobased δ-valerolactam monomer (3-(dimethylamino)-piperidone, ) derived from ornithine, which could efficiently copolymerize with ε-caprolactam to deliver functional nylon 6 polymers. Both monomer and copolymer synthesis proved straightforward and readily scalable. The resulting nylon 6 polymers exhibited comparable properties relative to their well-known commercial counterpart, including high thermal stability and crystallinity, suggesting that the incorporation of exerted minimal influence on the polymers’ inherent properties. Remarkably, the pendant dimethylamino group at the polyamide backbone could further convert into various functional structures by reacting with electrophiles, thereby providing a simple and versatile platform for the preparation of diverse functional nylon 6 materials towards broader applications.

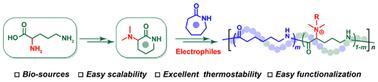
生物基δ-戊内酯与ε-己内酰胺开环共聚制备功能性尼龙6
ε-己内酰胺与功能内酰胺单体的共聚是在尼龙6中引入悬置取代基的有效策略,这可能会使这些材料具有更好的加工性能,溶解度,弹性和附着力,同时不影响其固有的热学和力学性能。然而,由于原料成本高和/或合成步骤繁琐,功能性内酰胺的规模化合成仍然非常困难,难以满足市场对尼龙6材料的巨大需求。本文中,我们引入了一种新的从鸟氨酸衍生的生物基δ-戊内酯单体(3-(二甲氨基)-哌啶酮,M1),该单体可以与ε-己内酰胺有效共聚,生成功能性尼龙6聚合物。单体和共聚物的合成都证明是简单的,易于扩展。由此产生的尼龙6聚合物表现出与其知名的商业对应物相当的性能,包括高热稳定性和结晶度,这表明M1的加入对聚合物的固有性能影响最小。值得注意的是,聚酰胺主链上的二甲氨基可以通过与亲电试剂反应进一步转化为各种功能结构,从而为制备各种功能尼龙6材料提供了一个简单而通用的平台,具有更广泛的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


