Interferon-responsive intestinal BEST4/CA7+ cells are targets of bacterial diarrheal toxins
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
BEST4/CA7+ cells of the human intestine were recently identified by single-cell RNA sequencing. While their gene expression profile predicts a role in electrolyte balance, BEST4/CA7+ cell function has not been explored experimentally owing to the absence of BEST4/CA7+ cells in mice and the paucity of human in vitro models. Here, we establish a protocol that allows the emergence of BEST4/CA7+ cells in human intestinal organoids. Differentiation of BEST4/CA7+ cells requires activation of Notch signaling and the transcription factor SPIB. BEST4/CA7+ cell numbers strongly increase in response to the cytokine interferon-γ, supporting a role in immunity. Indeed, we demonstrate that BEST4/CA7+ cells generate robust CFTR-mediated fluid efflux when stimulated with bacterial diarrhea-causing toxins and find the norepinephrine-ADRA2A axis as a potential mechanism in blocking BEST4/CA7+ cell-mediated fluid secretion. Our observations identify a central role of BEST4/CA7+ cells in fluid homeostasis in response to bacterial infections.
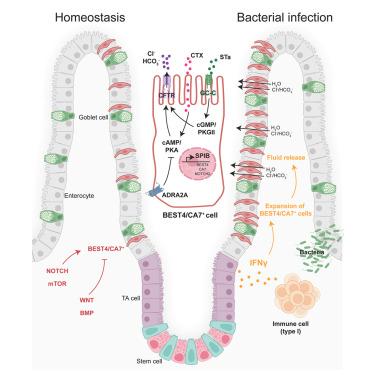
干扰素反应性肠道BEST4/CA7+细胞是细菌性腹泻毒素的靶标
人类肠道的BEST4/CA7+细胞最近被单细胞RNA测序鉴定。虽然它们的基因表达谱预测了电解质平衡的作用,但由于小鼠中缺乏BEST4/CA7+细胞和缺乏人体外模型,尚未对BEST4/CA7+细胞的功能进行实验探索。在这里,我们建立了一个允许在人类肠道类器官中出现BEST4/CA7+细胞的方案。BEST4/CA7+细胞的分化需要激活Notch信号和转录因子SPIB。对细胞因子干扰素-γ的反应中,BEST4/CA7+细胞数量强烈增加,支持免疫作用。事实上,我们证明了BEST4/CA7+细胞在受到引起腹泻的细菌毒素刺激时产生强大的cftr介导的液体流出,并发现去甲肾上腺素- adra2a轴是阻断BEST4/CA7+细胞介导的液体分泌的潜在机制。我们的观察确定了BEST4/CA7+细胞在应对细菌感染的体液稳态中的核心作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


