Dynamic PRDX S-acylation modulates ROS stress and signaling
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Peroxiredoxins (PRDXs) are a highly conserved family of peroxidases that serve as the primary scavengers of peroxides. Post-translational modifications play crucial roles modulating PRDX activities, tuning the balance between reactive oxygen species (ROS) signaling and stress. We previously reported that S-acylation occurs at the “peroxidatic” cysteine (Cp) site of PRDX5 and that it inhibits PRDX5 activity. Here, we show that the PRDX family more broadly is subject to S-acylation at the Cp site of all PRDXs and that PRDX S-acylation dynamically responds to cellular ROS levels. Using activity-based fluorescent imaging with DPP-Red, a red-shifted fluorescent indicator for acyl-protein thioesterase (APT) activity, we also discover that the instigation of ROS-stress via exogenous H2O2 activates both the cytosolic and mitochondrial APTs, whereas epidermal growth factor (EGF)-stimulated endogenous H2O2 deactivates the cytosolic APTs. These results indicate that APTs help tune H2O2 signal transduction and ROS protection through PRDX S-deacylation.
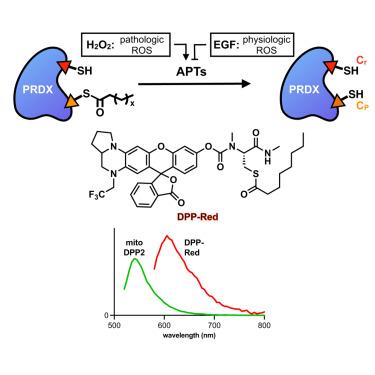

动态PRDX s -酰化调节ROS应激和信号传导
过氧化物还毒素(PRDXs)是一种高度保守的过氧化物酶家族,是过氧化物的主要清除剂。翻译后修饰在调节PRDX活性,调节活性氧(ROS)信号和应激之间的平衡中起着至关重要的作用。我们之前报道过s -酰化发生在PRDX5的“过氧化物”半胱氨酸(Cp)位点,并抑制PRDX5的活性。在这里,我们发现PRDX家族更广泛地受到所有PRDX的Cp位点s -酰化的影响,并且PRDXs -酰化动态响应细胞ROS水平。利用DPP-Red(一种用于检测酰基蛋白硫酯酶(APT)活性的红移荧光指示剂)的活性荧光成像,我们还发现,外源性H2O2诱导的ros胁迫激活了胞质和线粒体的APT,而表皮生长因子(EGF)刺激的内源性H2O2使胞质内的APT失活。这些结果表明,APTs通过PRDX s -去酰化调节H2O2信号转导和ROS保护。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


