Mechanisms and effects of gas intercalation into ionic liquids confined within charged nanoscale volumes
IF 5.8
3区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
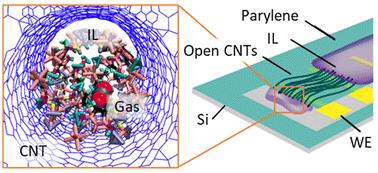
Understanding the behavior of gas within confined ionic liquids (ILs) is important for a wide range of emerging energy, separation, and sensing technologies. However, the mechanisms governing gas solubility and molecular structure within these systems remain largely unknown. Here, we investigate the factors that dictate the intercalation and arrangement of CO2, N2 and O2, in a commonly used IL (1-butyl-3-methylimidazolium hexafluorophosphate, [BMIM+][PF6−]) confined within neutral and charged 2.1 nm diameter carbon nanotubes (CNTs) via molecular dynamics simulations and enhanced free energy sampling methods. Our simulations show that the gas selectivity in these systems can be explained by a competitive complex interplay between confinement, charge state of CNTs, and IL properties. We then experimentally validate a subset of these predictions using a novel device consisting of electrically addressable, IL-infilled CNTs which we expose to CO2 and O2 in a N2 background. Our findings help to disentangle the relative importance of tuning gas solubility and preferential proximity to the CNT wall for maximizing measurable changes of electrochemical signals. These insights provide a foundation for engineering future electrochemical systems utilized in gas sensing or separation applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nanoscale
CHEMISTRY, MULTIDISCIPLINARY-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
12.10
自引率
3.00%
发文量
1628
审稿时长
1.6 months
期刊介绍:
Nanoscale is a high-impact international journal, publishing high-quality research across nanoscience and nanotechnology. Nanoscale publishes a full mix of research articles on experimental and theoretical work, including reviews, communications, and full papers.Highly interdisciplinary, this journal appeals to scientists, researchers and professionals interested in nanoscience and nanotechnology, quantum materials and quantum technology, including the areas of physics, chemistry, biology, medicine, materials, energy/environment, information technology, detection science, healthcare and drug discovery, and electronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


