Boron-Induced Redispersion of Pt Species during Propane Dehydrogenation
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In a propane dehydrogenation (PDH) reaction system, low-Pt catalysts generally suffer from rapid deactivation and poor durability due to easy sintering at high temperatures and in a reductive atmosphere. Herein, we develop a catalyst (PtSnK-B/Al2O3, named as PtSnK-B0.32) with both low Pt loading (0.15 wt %) and high durability by facile doping of trace boron into a conventional Pt-based catalyst. Density functional theory (DFT) calculations show that pure Pt clusters have weak binding energy with support, leading to a further undesired Pt sintering process. In contrast, when boron (B) is added to the Pt-based catalyst, the undesired Pt sintering process is significantly inhibited. Moreover, being initiated by propane molecules, the pure Pt clusters are readily to dissociate into Pt atoms due to their longer Pt-Pt bond lengths, then the dissociated Pt atoms are captured by B or BOx species to form stable Pt–B clusters under PDH conditions. The formation of highly dispersed Pt–B clusters allows the catalyst to achieve high intrinsic activity; compared with the catalyst without B (PtSnK, 0.14 wt % Pt), the Ea value of the B-doped catalyst is obviously reduced. Significantly, the durability of PtSnK-B0.32 is three times that of PtSnK and even twice that of 0.26PtSnK with a Pt loading of 0.26 wt %. The facile synthesis method, lower Pt content, and higher durability provide a promising application perspective.
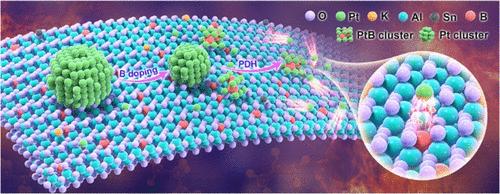
丙烷脱氢过程中硼诱导的铂类再分散
在丙烷脱氢(PDH)反应体系中,由于在高温和还原气氛中容易烧结,低铂催化剂通常失活快,耐久性差。在此,我们开发了一种催化剂(PtSnK-B/Al2O3,命名为PtSnK-B0.32),通过在传统的Pt基催化剂中轻松掺杂微量硼,具有低Pt负载(0.15 wt %)和高耐久性。密度泛函理论(DFT)计算表明,纯Pt团簇与载体的结合能较弱,导致Pt烧结过程进一步不理想。相反,当硼(B)添加到Pt基催化剂中时,不希望的Pt烧结过程被显著抑制。此外,在丙烷分子的引发下,纯Pt簇由于其较长的Pt-Pt键长,容易解离成Pt原子,然后解离的Pt原子被B或BOx捕获,在PDH条件下形成稳定的Pt- B簇。形成高度分散的Pt-B簇,使催化剂具有较高的本构活性;与不含B的催化剂(PtSnK, 0.14 wt % Pt)相比,B掺杂催化剂的Ea值明显降低。值得注意的是,当Pt载荷为0.26 wt %时,PtSnK- b0.32的耐久性是PtSnK的3倍,甚至是0.26PtSnK的2倍。合成方法简单,铂含量低,耐久性好,具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


