Enantioselective total syntheses of melotenine-, voacafrine-, and tabersonine-type Aspidosperma indole alkaloids
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
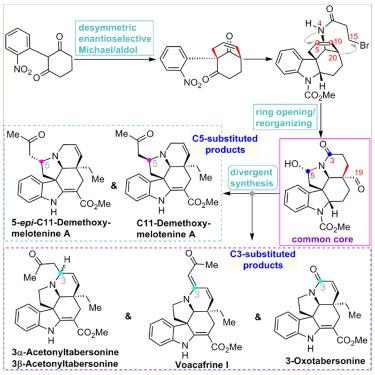
Although the total synthesis of aspidosperma natural products has been extensively investigated, the members possessing diverse functionalities at C3 and/or C5, which display much stronger bioactivity than the non-functionalized congeners, have not been accomplished due to the structural challenges. Herein, the first catalytic asymmetric total syntheses of C11-demethoxymelotenine, 3-oxotabersonine, voacafrine I, and 3α/3β-acetonyltabersonine are presented. Our synthesis hinged on the development of an organocatalyzed desymmetric enantioselective Michael/aldol reaction of 2-(2-nitrophenyl)cyclohexane-1,3-dione for the efficient construction of a chiral [3.3.1]-bridged bicyclic scaffold and a ring opening-reorganizing tactic of the bridged bicycle for elegant construction of a 6-5-6-5-6 pentacyclic ring system bearing functionalities amenable to distinguishable elaborations at C3, C5, and C19 positions. Utilizing the strategic polycyclic core, the enantioselective total syntheses of six previously unconquered aspidosperma-type alkaloids and stereoisomers have been achieved via a divergent manner through appropriately manipulating the functional groups at C3, C5, and C19 at the late stage.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


