Engineering d-band center of Mn to strengthen Mn–O bonding for long cycle life zinc-ion battery
IF 11.2
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
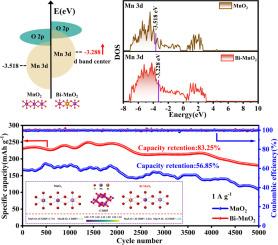
MnO2 has emerged as one of the favored cathode materials for aqueous zinc ion batteries (AZIBs) due to its high theoretical capacity and abundant crystalline structures. However, MnO2 cathode generally suffers from poor electrical conductivity and rapid capacity degradation due to unavoidable manganese dissolution during cycling, limiting their further utilization. In this study, we modify the d-band center of Mn by introducing non-precious metal Bi atoms into the MnO2 system, thereby strengthening the Mn–O bonding to inhibit manganese dissolution. Theoretical calculations reveal that the d-band center of Mn in Bi-MnO2 shifts upward, promoting electron transfer from O 2p orbitals to Mn–O bonding orbitals. This enhances the Mn–O bond strength, stabilizing Mn atoms in the crystal lattice and reducing manganese solvation loss. As a result, the conductivity and cyclic stability of Bi-MnO2 are significantly improved. The results demonstrate that Bi-MnO2 exhibits outstanding electrochemical properties, with a capacity of 392.3 mAh g−1 after 100 cycles at 0.2 A g−1 and a capacity retention of 83.25% after 5000 cycles at 1.0 A g−1. This study presents a new approach to address the manganese dissolution issue, which could further advance the application of d-band center theory in MnO2 materials.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


