MicroRNA control of stem cell reconstitution and growth in root regeneration
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
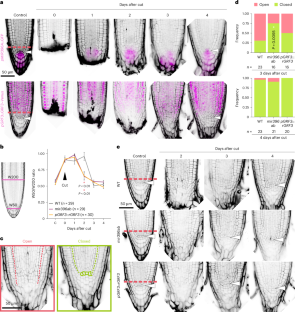
Plants display a remarkable regeneration capacity, which allows them to replace damaged or lost cells, tissues and organs, and thus recover from a broad spectrum of injuries1,2. Even lost stem cells can be regenerated from non-stem cells after competence acquisition, highlighting the enormous plasticity of plant cells. However, the molecular mechanisms underlying this process are still poorly understood. In the root, the highly conserved microRNA miR396 and its targets, the GROWTH-REGULATING FACTORs (GRFs), control the transition from stem cells to proliferative cells. miR396 promotes stem cell activity by repressing and excluding the GRFs from the stem cell area. In turn, the GRFs promote cell division in the proliferation zone3. Here we show that the miR396–GRF regulatory module guides stem cell reconstitution after root tip excision, playing a dual role: while miR396 promotes competence, the GRFs control regeneration speed. Moreover, plants with ectopic miR396 expression have defined stem cell niches before the excision but do not reconstitute them afterwards, remaining in an open state despite continuing to grow. We propose that this phenomenon is caused by dispersed stem cell activity, which supports growth after root tip excision without reconstituting the organized and spatially restricted stem cell niche typical of Arabidopsis roots. The miR396–GRF module controls a trade-off between competence and speed during root tip regeneration in Arabidopsis. Roots ectopically expressing miR396 grow without stem cell niche reconstitution, probably due to dispersed stem cell activity.

根再生过程中干细胞重建与生长的MicroRNA调控
植物显示出非凡的再生能力,这使它们能够替换受损或丢失的细胞、组织和器官,从而从各种各样的伤害中恢复过来。即使失去的干细胞也可以在获得能力后由非干细胞再生,这凸显了植物细胞的巨大可塑性。然而,这一过程背后的分子机制仍然知之甚少。在根中,高度保守的microRNA miR396及其靶标生长调节因子(GROWTH-REGULATING FACTORs, GRFs)控制着干细胞向增殖细胞的转变。miR396通过抑制和排除干细胞区域的grf来促进干细胞活性。反过来,GRFs促进增殖区的细胞分裂3。本研究表明,miR396 - grf调控模块指导根尖切除后的干细胞重建,发挥双重作用:miR396促进能力,grf控制再生速度。此外,异位miR396表达的植物在切除前已经定义了干细胞壁龛,但在切除后不会重建它们,尽管继续生长,但仍保持开放状态。我们认为这种现象是由分散的干细胞活性引起的,这种活性支持根尖切除后的生长,而不会重建有组织和空间限制的典型的拟南芥根系干细胞生态位。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


