Design, synthesis and optimization of Apcin analogues as Cdc20 inhibitors for triple-negative breast cancer therapy
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
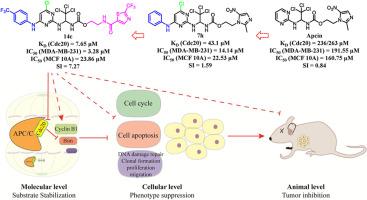
Cell division cycle 20 homologue (Cdc20) is an essential mitotic regulator whose overexpression is closely associated with tumorigenesis and poor prognosis in triple-negative breast cancer (TNBC). Targeting Cdc20 has therefore emerged as a promising therapeutic avenue for this aggressive malignancy. In the present study, a receptor-based drug design approach was employed to optimize Apcin analogues as Cdc20 inhibitors. Through a two-step strategy—concept validation followed by structural optimization—we identified compound 14c, which demonstrated remarkable Cdc20 binding affinity (KD: 7.65 μM), potent antiproliferative effects against MDA-MB-231 TNBC cells (IC50: 3.28 μM), and a favorable selectivity index (4.22 for MCF-7 non-TNBC cells and 7.27 for MCF 10A normal cells). 14c effectively inhibited Cdc20 activity, induced G2/M phase arrest, promoted DNA damage accumulation, and stabilized key substrates such as Cyclin B1 and Bim, leading to enhanced apoptosis and suppression of tumor cell proliferation and migration. In vivo, 14c significantly inhibited tumor growth in an MDA-MB-231 xenograft model with a 90 % tumor inhibition rate and no observable toxicity. These results highlight the potential of 14c as a potent Cdc20 inhibitor, offering a promising therapeutic approach for TNBC.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


