Synthesis and in vitro evaluation of radioiodine labeled hypoxia-targeted drugs containing 2-nitroimidazole and benzenesulfonamide groups
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
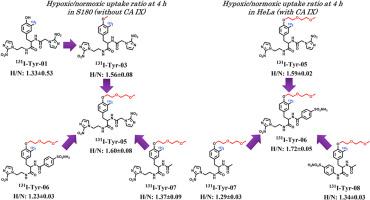
Designing new radiolabeled hypoxia-targeted drugs is of great help in the diagnosis of tumors. Hypoxia-targeted drugs with dual bioactive groups can enhance hypoxia selectivity, strengthen the binding of drugs to targets, and improve diagnostic accuracy compared with traditional hypoxia-targeted drugs containing only one nitroimidazole group. In this study, a series of novel radioiodine labeled tyrosine derivatives containing 2-nitroimidazole and benzenesulfonamide groups were synthesized and in vitro evaluated. In the uptake experiments of S180 cells that didn't express carbonic anhydrase IX (CAIX), the compound [131I]-3-(3-iodo-4-(2-(2-methoxyethoxy)ethoxy)phenyl)-2-(2-(2-nitro-1H-imidazole-1-yl)acetamido)-N-(2-(2-nitro-1H-imidazole-1-yl)ethyl)propenamide (131I-Tyr-05) containing two 2-nitroimidazole groups was modified from phenolic hydroxyl to methoxy to 2-(2-methoxyethoxy)ethoxy, gradually achieving improved membrane permeability and enhanced hydrophilicity. Compared with other compounds with similar structures but containing only one 2-nitroimidazole, it had higher hypoxic selectivity. In the uptake experiment of HeLa cells that expressed CAIX, [131I]-N-(3-(3-iodo-4-(2-(2-methoxyethoxy)ethoxy)phenyl)-1-((2-(2-nitro-1H-imidazole-1-yl)ethyl)amino)-1-oxopropan-2-yl)-4-sulfamoylbenzamide (131I-Tyr-06), which contained both 2-nitroimidazole and benzenesulfonamide group, achieved enhanced hypoxic uptake and selectivity through the combination of two targeting groups. The S180 cell blocking experiments of 131I-Tyr-05 and 131I-Tyr-06 showed that the benzenesulfonamide group of the compounds didn't inhibit cellular uptake, and inhibition of cytochrome P450 (CYP450) enzyme had no effect on cellular uptake. In silico ADMET evaluation showed that I-Tyr-05 and I-Tyr-06 possessed acceptable physicochemical and ADMET properties. In conclusion, this work demonstrated the advantages of hypoxia-targeted drugs containing dual bioactive groups compared to a single group, and also found it was a feasible approach to design new dual-targeted drugs by combining 2-nitroimidazole and benzenesulfonamide groups.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


