Development of an Innovative Three-Component Optical Resolution and a Flow Chemistry Process for (R)-Troloxamide Quinone (EPI-589)
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
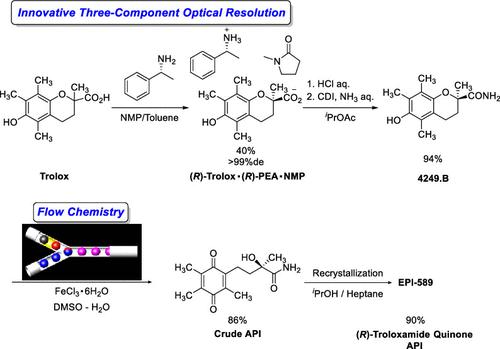
(R)-Troloxamide quinone (EPI-589) is a compound that is under development as a therapeutic agent for Parkinson’s disease and amyotrophic lateral sclerosis (ALS). The compound is derived from Trolox, a vitamin E derivative, using optical resolution and subsequent chemical conversion processes to obtain the active pharmaceutical ingredient (API). However, in the initially developed manufacturing method, pseudoephedrine was used as the optical resolution reagent, although it is restricted for use as a stimulant drug raw material in several countries, and the reproducibility of the optical resolution was not high. Thus, it was necessary to find an alternative chiral amine, which would be more freely usable and give high reproducibility. Among several optical resolution conditions investigated, an unusual three-component complex between (R)-Trolox, (R)-phenylethylamine, and N-methyl-2-pyrrolidone (NMP) was identified as most effective. However, although the optical resolution process showed both high reproducibility and optical purity, the manufacturing method came with the risk of introducing nitrosamines into the active pharmaceutical ingredient, so further extensive investigations were conducted to address this issue. As a result, a flow chemistry process was developed, which could avoid the use of a nitrate reagent in the oxidation step, and thus eliminate the risk of nitrosamine generation. This paper discloses the process development studies and discusses the successful manufacturing of EPI-589 on a scale of several hundred kilograms, utilizing the novel optical resolution and flow chemistry process under GMP conditions.
(R)-Troloxamide醌(EPI-589)创新三组分光学分辨及流动化学工艺的建立
(R)-Troloxamide quinone (EPI-589)是一种正在开发中的化合物,可作为帕金森病和肌萎缩侧索硬化症(ALS)的治疗剂。该化合物由维生素E衍生物Trolox衍生而来,通过光学分解和随后的化学转化过程获得活性药物成分(API)。然而,在最初开发的制造方法中,伪麻黄碱被用作光学分辨试剂,尽管它在一些国家被限制作为兴奋剂药物原料使用,并且光学分辨的重现性不高。因此,有必要寻找一种可替代的手性胺,这种手性胺可以更自由地使用并具有较高的重现性。在研究的几种光学分辨率条件中,(R)-Trolox, (R)-苯乙胺和n -甲基-2-吡咯烷酮(NMP)之间的一种不寻常的三组分配合物被认为是最有效的。然而,尽管光学分辨工艺具有高再现性和光学纯度,但该制造方法存在将亚硝胺引入活性药物成分的风险,因此进行了进一步的广泛研究以解决这一问题。因此,开发了一种流动化学工艺,可以避免在氧化步骤中使用硝酸盐试剂,从而消除亚硝胺生成的风险。本文介绍了在GMP条件下,利用新型光学分辨率和流动化学工艺成功生产EPI-589的工艺开发研究和讨论。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


