Comprehensive Synthetic Route Redesign of AZD5991: A High-Complexity Atropisomeric Macrocycle
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
We describe our approach to the total synthesis of AZD5991 (1) from a process development perspective through the complete redesign of our synthetic strategy from the ground up. The size and complexity of small-molecule therapeutic targets have continued to increase over recent decades. One such example, 1, is arguably the most complex active pharmaceutical ingredient (API) in AstraZeneca’s small molecule development portfolio to date and poses formidable synthetic challenges. The previous racemic synthesis of 1 was sufficient to supply early clinical activities; however, the route was not deemed commercially viable and had significant environmental challenges. The identification of a long-term sustainable route was therefore critical to enable the robust manufacture of drug substance for later clinical activities and launch. We report exploration of asymmetric approaches toward the atropisomeric core, new routes toward each of the four heterocyclic building blocks, including a divergent pyrazole functionalization, and final assembly in a scalable and controlled macrocyclization process. These improvements resulted in a 49% reduction in step count and 95% reduction in projected waste generation.
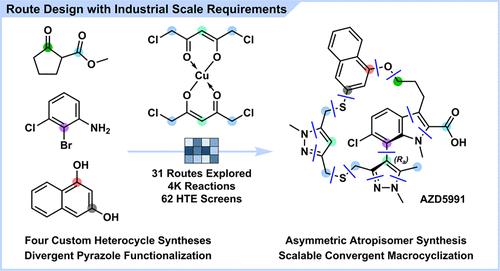
AZD5991合成路线的再设计:一个高复杂度的atrosom异构大环
我们从工艺开发的角度描述了我们的AZD5991(1)的全合成方法,通过从头开始的合成策略的完全重新设计。近几十年来,小分子治疗靶点的大小和复杂性不断增加。其中一个例子,1,可以说是迄今为止阿斯利康小分子开发组合中最复杂的活性药物成分(API),并且构成了艰巨的合成挑战。先前的外消旋合成1足以提供早期临床活性;然而,这条路线被认为在商业上不可行,并且存在重大的环境挑战。因此,确定一条长期可持续的路线对于能够为以后的临床活动和上市强有力地生产原料药至关重要。我们报告了对atro异构核心的不对称方法的探索,对四个杂环构建块中的每一个的新途径,包括发散吡唑功能化,以及在可扩展和可控制的大环化过程中的最终组装。这些改进导致步数减少49%,预计废物产生量减少95%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


