Organomolecular Ferroelectric Nanocatalyst Augments Tumor Immunotherapy by Inducing Apoptosis and Ferroptosis
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
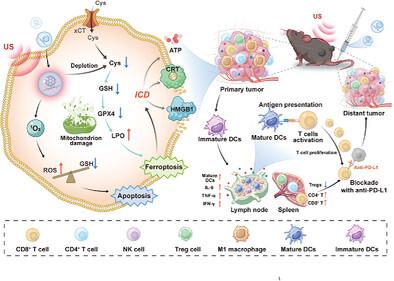
Immunogenic programmed cell death effectively triggers acute inflammatory responses, thereby enhancing antitumor immunity. The advancement of biodegradable nonmetallic dual inducers represents a promising strategy. Herein, a biodegradable organomolecular ferroelectric nanoplatform (C60-TCNQ, CT) is designed to facilitate effective ferroelectric catalysis, thereby augmenting tumor immunotherapy through apoptosis and ferroptosis. CT-mediated ultrasound-triggered ferroelectric catalysis promotes ferroelectric polarization and significantly increases the production of reactive oxygen species, leading to substantial tumor cell apoptosis. Moreover, the polycyano group of CT nanoparticles selectively reacts with cysteine under mild conditions, resulting in redox imbalances and the accumulation of lipid peroxides, which contribute to the induction of ferroptosis in tumor cells. Additionally, the apoptosis and ferroptosis induced by CT stimulate immunogenic cell death progression, eliciting robust immune responses. In vivo evaluation using a bilateral tumor model demonstrates the capacity of CT to sensitize anti-PD-L1 therapy under ultrasound irradiation, achieving an impressive antitumor response rate of 96.2% against malignant melanoma and an 80% inhibition of tumor metastasis. RNA sequencing analysis revealed that treatment with CT resulted in a downregulation of gene signatures associated with the immune-related Jak-Stat signaling pathway. This study opens a novel avenue to developing organomolecular ferroelectric nanomedicines for effective tumor immunotherapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


