Modulating Electronic Structure and Mass Transfer Kinetics via Mo-Mo2C Heterostructure for Ampere-Level Hydrogen Evolution
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
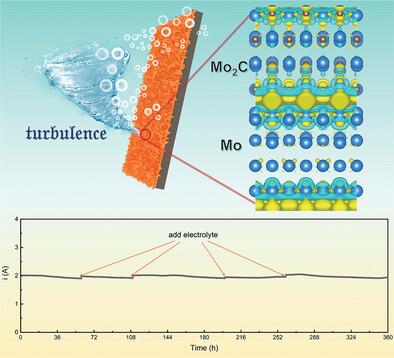
Molybdenum carbide (Mo2C), known for its platinum-like electronic structure and excellent corrosion resistance, has demonstrated promising catalytic performance in laboratory tests. However, under industrial harsh conditions, the catalytic performance of Mo2C faces constraints due to its inherently strong hydrogen adsorption. Additionally, at elevated current densities, rapid depletion of active species in the electrolyte, coupled with hydrogen gas bubble accumulation, introduce significant mass transport challenges. This work introduces an electrode with Mo-Mo2C heterostructures supported on a Mo plate (Mo-Mo2C/Mo). Further analyses reveal that incorporating metallic Mo into the heterostructures optimizes the electronic structure of Mo2C. This optimization achieves a more balanced hydrogen adsorption, while also enhancing the capacity for water adsorption and dissociation of Mo2C, collectively improving catalytic activity. Furthermore, this electrode features a unique “bush-like” surface morphology that can induce a “turbulence” effect in the electrolyte near the electrode surface, facilitating electrolyte flow and mass transport. As a result, the Mo-Mo2C/Mo electrode exhibits excellent catalytic performance at high current densities (η1000 = 452 mV). Moreover, the strong corrosion resistance and robust integration of Mo and Mo2C ensure long-term stability, with the electrode remaining stable at 1.5 A in 6 M KOH over extended periods.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


