Catalyst-Free Selective Synthesis of E-Tetrasubstituted Olefins via Tandem Reaction of 3-Acetyl-4-phenyl-1-oxaspiro[4.5]deca-3,6,9-triene-2,8-dione with Amine, C–C Bond Breakage, and Proton Transfer
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
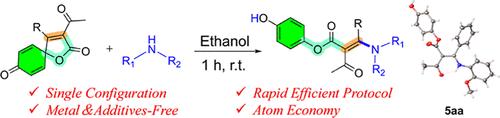
The Z- or E-selective syntheses of tetrasubstituted olefins present big challenges. Tremendous efforts are ongoing to overcome this issue, especially for acyclic structures. In this work, an E-stereoselective synthetic method of tetrasubstituted olefins through tandem reaction of 1,4-Michael addition of 3-acetyl-4-phenyl-1-oxaspiro[4.5]deca-3,6,9-triene-2,8-dione with amine, C–C bond breakage, and proton transfer by intermolecular hydrogen bonds was revealed with excellent atom economy and without catalysts and additives. A diverse set of E-tetrasubstituted olefins were obtained in 43% to 93% yields with excellent functional group tolerance for late-stage modifications of complex drug molecules. The reaction mechanism was proposed based on the deuterium-labeling experiment and density functional theory (DFT) calculation.
3-乙酰-4-苯基-1-氧阿斯皮罗[4.5]十-3,6,9-三烯-2,8-二酮与胺串联反应、C-C键断裂和质子转移的无催化剂选择性合成e-四取代烯烃
四取代烯烃的Z-或e -选择性合成面临着很大的挑战。人们正在进行巨大的努力来克服这个问题,特别是对于非循环结构。本研究通过1,4- michael加成3-乙酰-4-苯基-1-oxaspiro[4.5] -3,6,9-三烯-2,8-二酮与胺的串联反应、C-C键断裂和分子间氢键质子转移,揭示了一种具有优异原子经济性且无需催化剂和添加剂的四取代烯烃e-立体选择性合成方法。多种e-四取代烯烃的收率为43%至93%,对复杂药物分子的后期修饰具有良好的官能团耐受性。通过氘标记实验和密度泛函理论(DFT)计算,提出了反应机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


