Discovery of potent, highly selective, and orally bioavailable factor XIa inhibitors for anticoagulant therapy
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
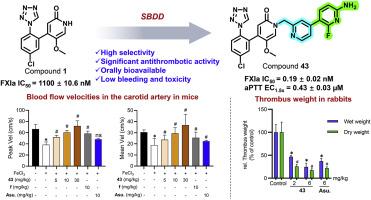
Factor XIa (FXIa) has emerged as a promising target for novel anticoagulant development since inhibiting it can reduce thrombosis without significant bleeding risks. Despite a few FXIa inhibitors entering clinical trials, none have been approved for the market yet. Here, we present highly selective and orally bioavailable FXIa inhibitors derived from compound 1, 4-(5-chloro-2-(1H-tetrazol-1-yl)phenyl)-5-methoxypyridin-2(1H)-one. Structure-activity relationship studies led to the discovery of promising 1-(pyridin-2-ylmethyl)pyridin-2(1H)-one-based FXIa inhibitors 37, 39b, 43, and 46b, which exhibited enhanced FXIa potency and selectivity compared to asundexian, an FXIa inhibitor in phase III clinical trials. Their anticoagulant activity was also comparable to or greater than that of asundexian. Compound 43 significantly reduced thrombosis in both FeCl3-induced mouse and rabbit arterial thrombosis models, demonstrating superior efficacy compared to asundexian. Importantly, 43 did not increase bleeding risks and exhibited a favorable safety profile in mice, suggesting its potential as a promising FXIa inhibitor for the treatment of thrombosis.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


