High-affinity, broad-spectrum, "centipede-like" multi-branched drug conjugates, anchored to the S protein, for blocking coronavirus infection
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
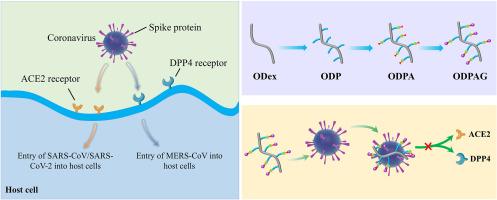
Over the past two decades, various coronaviruses have posed a severe threat to human life and health, with the spike protein (S protein) being a critical protein for infecting host cells. Glycyrrhizic acid (GA), as a natural drug, can inhibit the infection of coronaviruses by binding to the receptor-binding domain (RBD) of the S protein. However, issues like poor water solubility and weak binding affinity with the S protein have hindered its further application. Therefore, drawing inspiration from the biological structure of centipedes, a ROS-responsive multi-branched drug conjugate (ODPAG) was constructed through a "polymer-drug linkage" strategy using dextran as the backbone and GA as the active "claw". ODPAG exhibited drug loading of 22.0 ± 0.2% (OD40kPAG) and 19.7 ± 0.1% (OD450kPAG), showing ROS responsiveness with a half-life 6.4 times that of GA (OD40kPAG) and 5.4 times longer (OD450kPAG). In in vitro antiviral experiments, ODPAG exhibited an enhanced binding affinity to the S protein, with IC50 values of 1.33 μM (OD40kPAG) and 0.89 μM (OD450kPAG) against SARS-CoV-2 pseudovirus, demonstrating exceptional antiviral efficacy. These results collectively indicate that ODPAG can block coronavirus infection by binding to the S protein, exhibiting significant potential in addressing the current challenges posed by the novel coronavirus. Additionally, the "polymer-drug conjugate" strategy employed in this process is efficient, cost-effective, and offers new insights for combating future emergent coronaviruses.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


