MTHFR variant links homocysteine metabolism and endothelial cell dysfunction by targeting mitophagy in human thoracic aortic dissection patient induced pluripotent stem cell (iPSC) models
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Aims
Genetics and environmental cues boost the development of human diseases. Methylenetetrahydrofolate reductase (MTHFR) is involved in the metabolism of homocysteine, and a common variant rs1801133 of MTHFR has been reported in human cardiovascular diseases. This study aims to providing a novel strategy for patient stratification with specific genetic and metabolic screening, finally for personalized healthcare for patients with thoracic aortic dissection.Methods and results
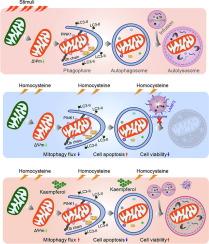
We corrected the MTHFR variant to generate an isogenic control iPSC line (Isogenic-iPSC) with CRISPR/Cas9 method, and this isogenic-iPSC shared the same other genetic information with our previously established MTHFR-iPSC line, providing a promising approach for analysis the phenotype and mechanism of rs1801133. During the direct differentiation of endothelial cells from both iPSC lines, rs1801133 variant did not affect the endothelial cell fate determination. Without homocysteine, this variant has little effect on endothelial cell function. While administration of homocysteine, the MTHFR-iPSC derived endothelial cells exhibited disrupted mitophagy, increased cell apoptosis and decreased cell viability. Bulk RNA-seq data indicated LAMP3 is a target of homocysteine, activation of LAMP3 might contribute to homocysteine induced the disruption of mitochondrial structure and cell apoptosis. With chemical compounds screening, kaempferol ameliorated the homocysteine-induced cell toxicity by restoring the mitochondrial structure. The direct relationship between homocysteine metabolism and MTHFR rs1801133 variant was investigated, and the molecular target for homocysteine and translational perspective has also been demonstrated.Conclusions
Collectively, this study provided the direct evidence of a specific genetic variant in MTHFR and homocysteine metabolism. Investigating the molecular mechanism of homocysteine activated LAMP3 on endothelial cell dysfunction and mitophagy could provide novel insights for targeted disease prevention and improving individual outcomes.TRANSLATIONAL PERSPECTIVE
Thoracic aortic dissection (TAD) is a life-threatening cardiovascular disease with a high mortality, lacking effective medical treatment and early diagnosis. Endothelial cells dysfunction has been considered into the development of TAD. Here, we show that MTHFR variant is responsible for the elevated homocysteine in iPSC-ECs, and disrupted mitochondrial structures by homocysteine significantly impaired endothelial function. Understanding the mechanism and translational medicine of homocysteine-induced endothelial toxicity in human with MTHFR variant could benefit the novel strategy for prevention and vessel protection against metabolism injury. Meanwhile, targeting mitophagy and application of small molecule, such as kaempferol, also provide an insight for endothelial protection.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


