Allylic Hydrogen Acidity of 1-Butene Derivatives Coordinated to Transition Metals─A Mechanistic Insight Including Carbonyl–Olefin Metathesis
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The coordination of organic molecules to transition metals significantly alters the electron density distribution, influencing the acidity of specific hydrogen atoms. This study scrutinizes the acidity of allylic hydrogens in transition metal-coordinated alkenes, delving into the factors that govern allylic proton abstraction. Employing density functional theory, we investigate the effects of various parameters, including the electronic nature of substituents on the vinylic carbons of the alkene, the oxidation state of the metal, and the identity of the transition metal center on the allylic hydrogens’ acidity. Our findings reveal that the impact on the acidity of allylic hydrogens in alkenes coordinated to gold(III), a third-row transition metal, is considerably substantial both kinetically and thermodynamically. Conversely, the impact is minimal for cobalt(III) from the first row and moderate for rhodium(III) from the second row of transition metals. Furthermore, our results indicate that electron-withdrawing substituents on vinylic carbons generally enhance the acidity of allylic hydrogens. The influence of oxidation state is also profound, as gold(I) exhibits markedly weaker effects compared to gold(III). To illustrate the practical application of these insights, we present a case study involving the use of AuCl3 to catalyze an organic transformation [Chem. Eur. J. 2020, 26, 1941–1946], elucidating the mechanism initiated by the deprotonation of the allylic hydrogen in the coordinated alkene.
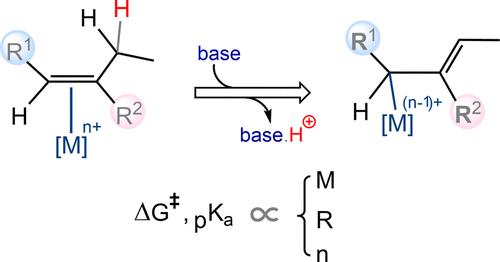
与过渡金属配位的1-丁烯衍生物的烯丙基氢酸─包括羰基-烯烃复分解的机理研究
有机分子与过渡金属的配位显著改变了电子密度分布,影响了特定氢原子的酸度。本研究考察了过渡金属配位烯烃中烯丙基氢的酸度,探讨了影响烯丙基质子抽离的因素。利用密度泛函理论,我们研究了各种参数对烯丙基氢酸度的影响,包括取代基在烯烃乙烯碳上的电子性质、金属的氧化态和过渡金属中心的身份。我们的研究结果表明,在与第三排过渡金属金(III)配位的烯烃中,烯丙基氢对酸性的影响在动力学和热力学上都是相当可观的。相反,对第一排钴(III)的影响最小,对第二排过渡金属铑(III)的影响中等。此外,我们的研究结果表明,乙烯基碳上的吸电子取代基通常会提高烯丙基氢的酸性。氧化态的影响也很深远,因为金(I)的影响明显弱于金(III)。为了说明这些见解的实际应用,我们提出了一个案例研究,涉及使用AuCl3催化有机转化[化学]。欧元。[j] . 2020, 26, 1941-1946],阐明了配位烯烃中烯丙基氢去质子化引发的机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


