Translating premalignant biology to accelerate non-small-cell lung cancer interception
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Over the past decade, substantial progress has been made in the development of targeted and immune-based therapies for patients with advanced non-small-cell lung cancer. To further improve outcomes for patients with lung cancer, identifying and intercepting disease at the earliest and most curable stages are crucial next steps. With the recent implementation of low-dose computed tomography scan screening in populations at high risk, there is an emerging unmet need for new diagnostic, prognostic and therapeutic tools to help treat patients suspected of harbouring premalignant lesions and minimally invasive non-small-cell lung cancer. Continued advances in the identification of the earliest drivers of lung carcinogenesis are poised to address these unmet needs. Employing multimodal approaches to chart the temporal and spatial maps of the molecular events driving lung premalignant lesion progression will refine our understanding of early carcinogenesis. Elucidating the molecular drivers of premalignancy is critical to the development of biomarkers to detect those incubating a premalignant lesion, to stratify risk for progression to invasive cancer and to identify novel therapeutic targets to intercept that process. In this Review, we summarize emerging insights into the earliest cellular and molecular events associated with lung squamous and adenocarcinoma carcinogenesis and highlight the growing opportunity for translating these insights into clinical tools for early detection and disease interception to transform the outcomes for those at risk for lung cancer. While progress has been made in treating advanced non-small-cell lung cancer, patients would further benefit from methods that detect progressive premalignant lesions and strategies that effectively intercept progression. In this Review, Mazzilli et al. explore recent advances in identifying premalignant lesions, highlighting their disease aetiology and devising approaches for stratification and interventions aimed at halting the progression to cancer.
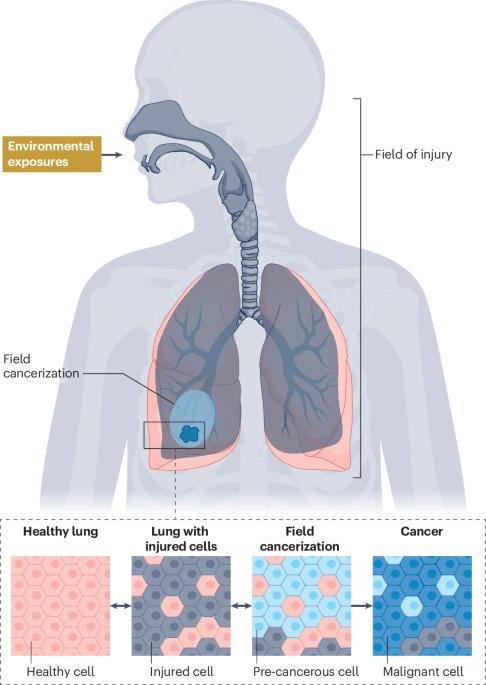
翻译癌前生物学加速非小细胞肺癌的拦截
在过去的十年中,针对晚期非小细胞肺癌患者的靶向和免疫疗法的开发取得了实质性进展。为了进一步改善肺癌患者的预后,在最早和最可治愈的阶段识别和拦截疾病是下一步的关键步骤。随着最近在高危人群中实施低剂量计算机断层扫描筛查,出现了对新的诊断、预后和治疗工具的需求,以帮助治疗疑似有癌前病变和微创非小细胞肺癌的患者。在确定肺癌发生的早期驱动因素方面的持续进展准备解决这些未满足的需求。采用多模态方法绘制驱动肺癌前病变进展的分子事件的时空图,将改进我们对早期癌变的理解。阐明恶性前病变的分子驱动因素对于开发生物标志物来检测恶性前病变的孵化、对进展为侵袭性癌症的风险进行分层以及确定新的治疗靶点来拦截这一过程至关重要。在这篇综述中,我们总结了与肺鳞状癌和腺癌相关的早期细胞和分子事件的新见解,并强调了将这些见解转化为早期检测和疾病拦截的临床工具以改变肺癌风险患者预后的机会越来越大。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


