Dendritic cell-based microrobots for enhanced systemic antigen-specific immune tolerance
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
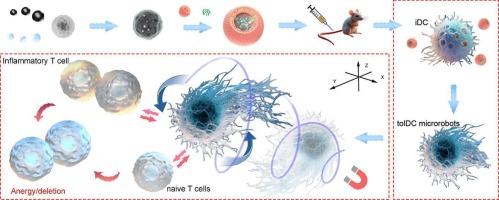
Current immunotherapeutic approaches for autoimmune disorders primarily rely on the use of generalized immunosuppressive medications. However, most immune drugs and tolerogenic immunomodulators are insufficient on their own to establish antigen-specific immunological tolerance (ASIT). Therefore, steering antigen-presenting cells (APCs) towards a tolerogenic state with minimal risk of broad immune suppression may be an effective approach. In pursuit of enhanced ASIT, magnetic nanoparticles cloaked with an erythrocyte membrane anchored with the model antigen ovalbumin have been successfully developed, allowing for the in vivo conversion of APCs into tolerogenic microrobots that respond to magnetic activation. Actuated by a rotating magnetic field (RMF), the in situ-formed cell-based microrobots can be guided to inflammatory sites, thereby augmenting systemic and local immune tolerance. These tolerogenic microrobots represent an innovative platform for active immunomodulation and provide precise control over the magnitude and direction of immune responses. This breakthrough offers new insights into the therapeutic management of allergies, autoimmune disorders, and the prevention of anti-drug antibodies in biologic therapies.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


